Mechanism + Description
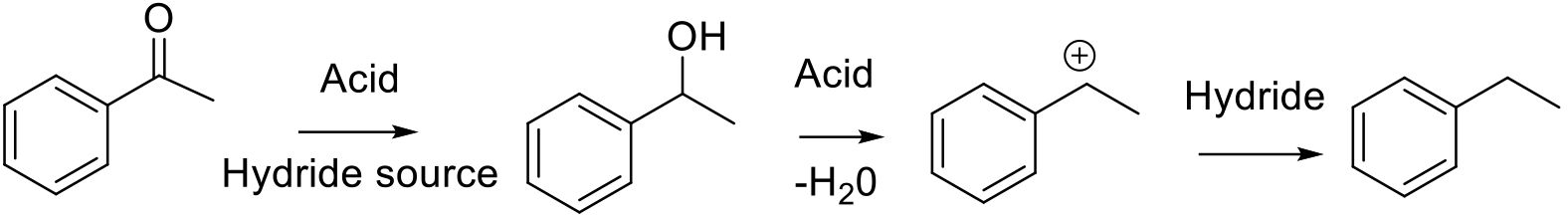
Typically from ketone activated by protonation then reduction to the alcohol. The acidic conditions produce a carbonium ion which is reduced to the hydrocarbon by another hydride donation. Some substrates may go through a radical mechanism.
General comments
In most ketone reductions, the alcohol is the desired product, but occasionally complete reduction (deoxygenation) to the hydrocarbon is desired. This transformation is not always readily feasible for all ketones, and generally substrates that can form stable carbonium ions are favoured. Typical reagents are protic acids and a silane source like Et3SiH. Ketone activation and carbonium ion formation can also be catalysed by Lewis acids. For some substrates catalytic hydrogenolysis can give the hydrocarbon from the ketone via the alcohol as an intermediate.
Other, older methodologies exist such as Wolff-Kishner Reduction (hydrazine/KOH) / Clemmensen Reduction (Zn/acid), but are rarely encountered these days.
Key references
Mayr, H.; Dogan, B. Selectivities in ionic reductions of alcohols and ketones with triethylsilane/trifluoroacetic acid. Tet. Letters 1997, 38, 1013-1016.
Larson, G. L.; Fry, J. L. Ionic and Organometallic‐Catalyzed Organosilane Reductions. Org. React. 2008, 71, 1-737.
Zotto, C. D.; Virieux, D.; Campagne, J.-M. FeCl3-Catalyzed Reduction of Ketones and Aldehydes to Alkane Compounds. Synlett 2009, 2, 276-278.
Nagai, Y. Hydrosilanes as reducing agents. A review. Org. Prep. Proc. Int. 1980, 12, 13-48.
Argouarch, G. Mild and efficient rhodium-catalyzed deoxygenation of ketones to alkanes. New J. Chem. 2019, 43, 11041-11044.
Fry, J. L.; Silverman, S. B.; Orfanopoulos, M. Reduction of Ketones to Hydrocarbons with Triethylsilane: m‐Nitroethylbenzene. In Organic Syntheses; John Wiley & Sons, Inc., 2003.
Grainger, D. M.; Zanotti-Gerosa, A.; Cole, K. P.; Mitchell, D.; May, S. A.; Pollock, P. M.; Calvin, J. R. Development of a Stepwise Reductive Deoxygenation Process by Ru‐Catalysed Homogeneous Ketone Reduction and Pd‐Catalysed Hydrogenolysis in the Presence of Cu Salts. ChemCatChem 2013, 5, 1205–1210.
Volkov, A.; Gustafson, K. P. J.; Tai, C-W.; Verho, O.; Bäckvall, J-E.; Adolfsson, H.Mild Deoxygenation of Aromatic Ketones and Aldehydes over Pd/C Using Polymethylhydrosiloxane as the Reducing Agent. Angew. Chem. Int. Ed. 2015, 54, 5122-5126.
Szmant, H. H. The Mechanism of the Wolff‐KishnerSzmant, H. H. Reduction, Elimination, and Isomerization Reactions. Angew. Chem. Int. Ed. 1968, 7 (2), 120-128.
Nakabayashi, T. Studies on the Mechanism of Clemmensen Reduction. I. The Kinetics of Clemmensen Reduction of p-Hydroxyacetophenone. J. Am. Chem. Soc. 1960, 82, 3900–3906.
Relevant scale up examples
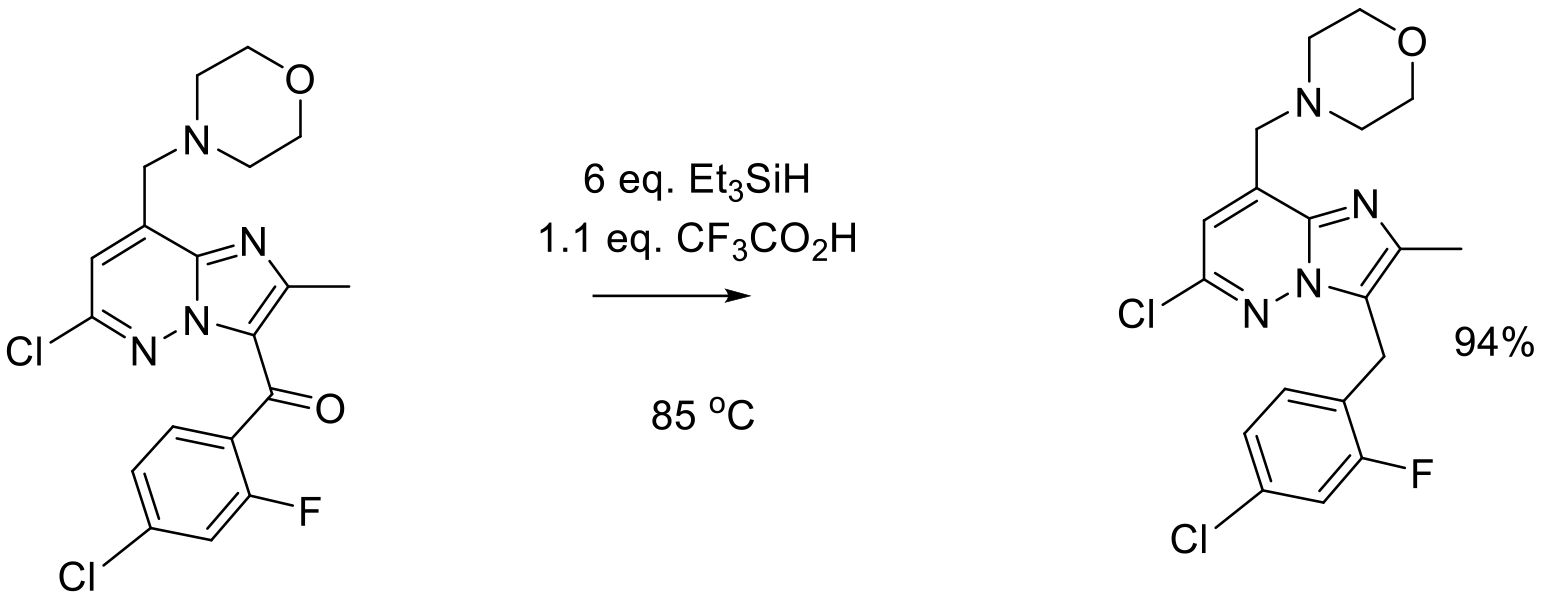
Org. Proc. Res. Dev. 2012, 16, 70–81.
44 kg scale
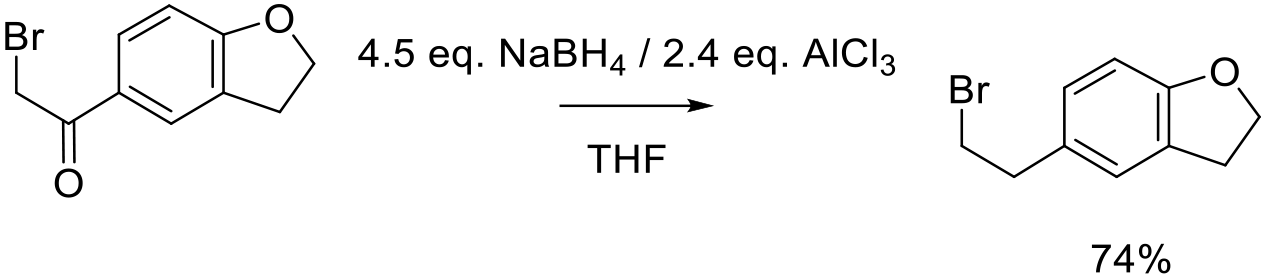
Org. Proc. Res. Dev. 2012, 16, 1591−1597.
100 g scale


