Reduction with Hydride Reagents
Mechanism + Description
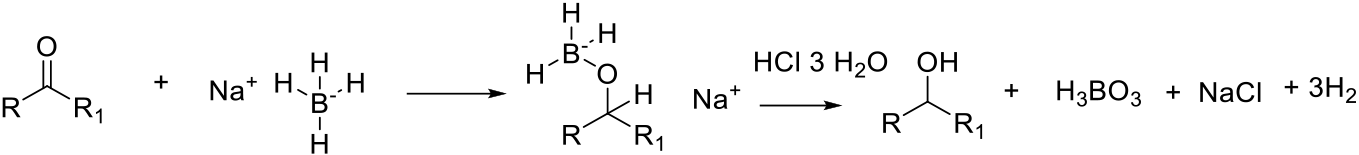
Transfer of a hydride to the electrophilic ketone to give an anionic ‘ate complex. More than one hydride can be transferred. Depending on the nature of the Al/B reagent, pre-coordination to the ketone may provide additional activation for hydride addition. Quenching with water/aqueous acid liberates the alcohol product.
General comments
Most commonly used boron and aluminium hydride reagents will reduce ketones to alcohols, although in practice, owing to the favourable reduction potential of the ketone group, sodium borohydride is often the agent of choice giving reasonable atom economy, good economics and ease of handling on scale. NaBH4 is available as a solid or in solution in basic water or certain organic solvents.
It is rare to have to use more powerful reductants like LiAlH4 or Red-Al™ to reduce most ketones, although in some cases (diastereomeric reduction) reagents like l-selectide or aluminium hydride reagents are encountered.
Key references
Brown, H. C.; Krishnamurthy, S. Forty years of hydride reductions. Tetrahedron 1979, 35, 567-607.
Relevant scale up examples
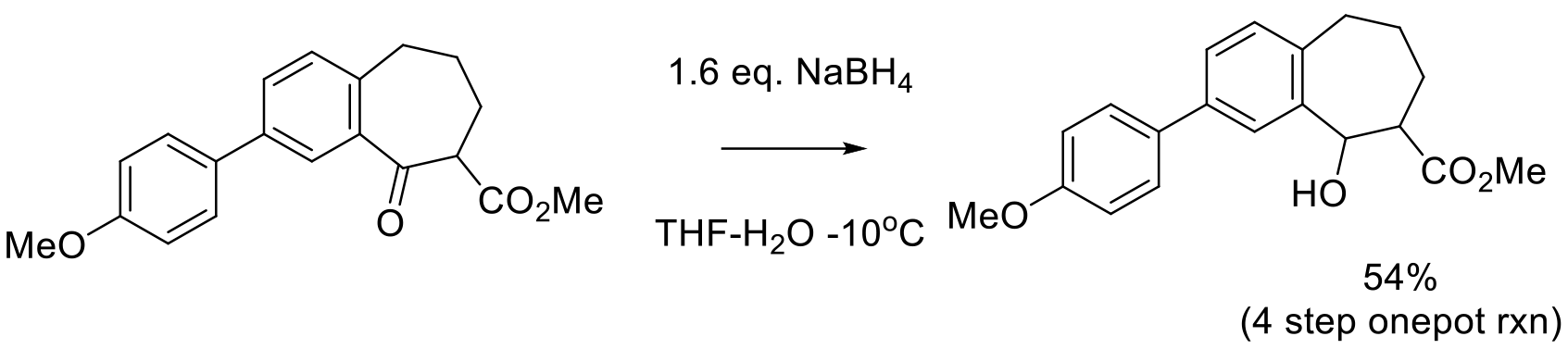
Org. Proc. Res. Dev. 2000, 4, 520-525.
8 kg scale
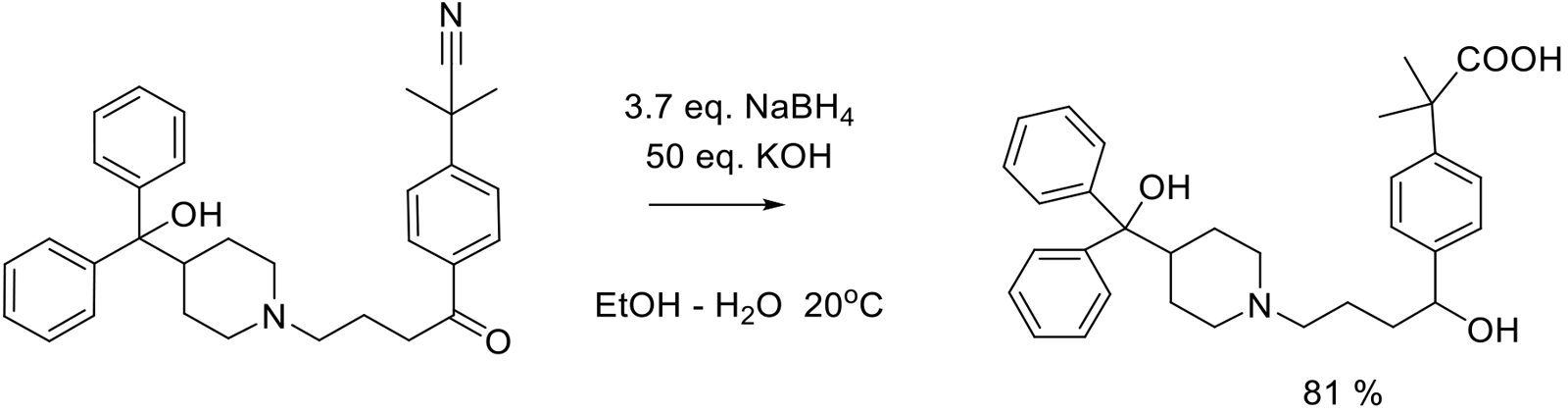
Org. Proc. Res. Dev. 2010, 14, 1464–1468.
500 g scale
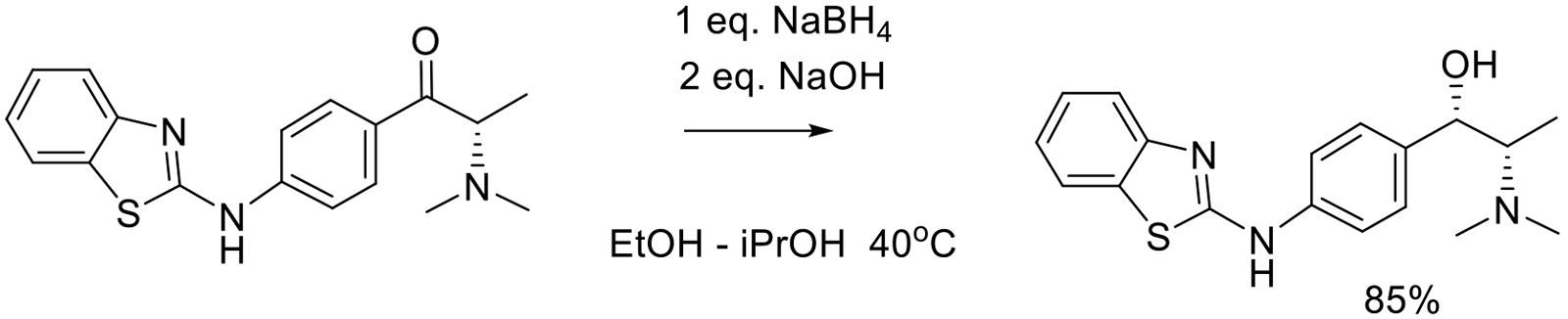
Org. Proc. Res. Dev. 2001, 5, 467-471.
120 g scale
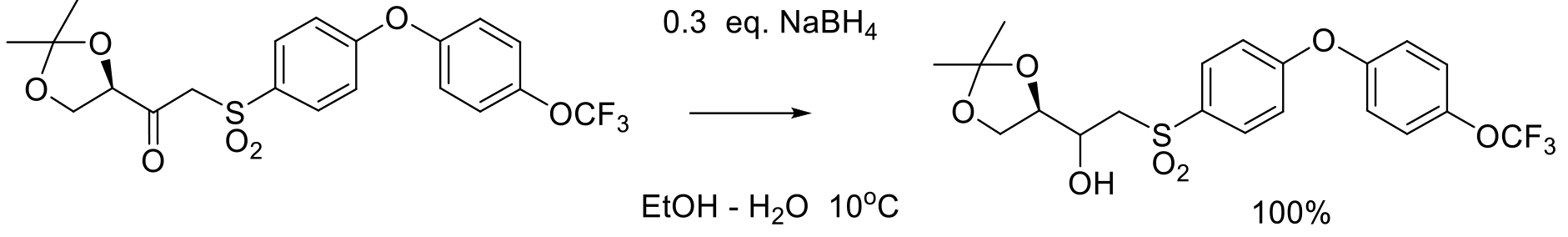
Org. Proc. Res. Dev. 2002, 6, 329-335.
12 kg scale
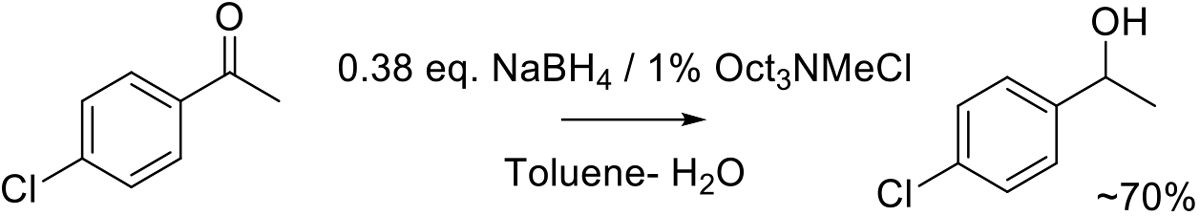
Org. Proc. Res. Dev. 2012, 16, 1279−1282.
150 kg scale
Green Review
-
Atom efficiency (by-products, molecular weight)
Atom efficiency depends on how many hydride equivalents are needed and can be utilized per mole of reducing agent. For instance, NaBH4 has four hydrides that can be transferred, although in practice it can be difficult to use all four. The more hydrides that can be utilized per mole of reducing agent, the better the atom efficiency. - Safety Concerns
Most boron and aluminium hydride reagents are pyrophoric and will liberate flammable hydrogen gas on quenching. H2 will form an explosive mixture in the presence of air. Reagents or combinations of reagents that could form B2H6 need special caution since this can form in the headspace above reactions and present an explosion hazard. - Toxicity and environmental/aquatic impact
There may be issues with discharging aqueous waste with high aluminium and boron content. Emerging data suggest boron compounds maybe more ecotoxic than previously thought. - Cost, availability & sustainable feedstocks
Materials for simple hydride reagents like NaBH4 and LiAlH4 are readily available and cheap. More complex hydride reagents tend to be more expensive. There is little opportunity for use of sustainable feedstocks. - Sustainable implications
All metals have a high LCA impact from mining and refining operations, so use should be catalytic with recovery and recycle. Aluminum is currently considered at low risk of depletion and boron is listed as limited availability with future risk to supply. Recycling may not be economically feasible.