Oxidation with Stoichiometric Oxidants
Mechanism + Description
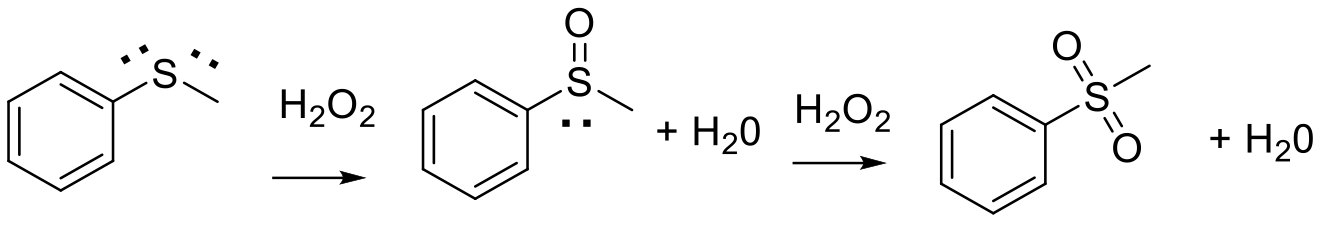
Usual mechanism via nucleophilic attack of sulfur lone pair on an electrophilic oxygen source.
General comments
The conventional route to synthesis sulfoxides and sulfones is via stoichiometric oxidation with a variety of oxidizing agents. Traditionally a range of heavy metal based oxidants would have been used (e.g. Ce, Mn, Cr), but these have now mainly been replaced with greener, less toxic and polluting reagents and catalytic methods. The differentiation between SO and SO2 is determined via choice of oxidant, solvent, design of reaction conditions and additives/catalysts.
Typical stoichiometric reagents used are:
- O2
- H2O2 / Urea H2O2
- NaOCl/NaOCl:5H20
- Organic peroxides/hydroperoxides such as t-BuOOH, Dioxiranes,
- Organic peracids such as CH3CO3H/MCPBA
- Inorganic peracids and salts such as Potassium peroxymonosulfate triple salt 2KHSO5·KHSO4·K2SO4 (Oxone™)/ perborate/magnesium monoperoxyphthalate (MMPP)
- Organic and inorganic hypervalent iodine compounds
- High oxidation state metal-based reagents, KMnO4, CrO3 / (Cr2O7)2-reagents, Ceric ammonium nitrate – (NH4)2Ce(NO3)6
Many of the more traditional oxidants like Oxone™ and MMPT used for sulfur oxidation have poor atom economy, although the by-products are reasonably benign. Oxidation is a thermodynamically favorable process and due concern needs to be given to controlling any reaction exotherm and avoiding generation of hazardous reaction mixtures and residues.
Heavy metal -based reagents based on Mn, Cr and Ce are rarely used now. They are often heterogeneous, highly exothermic and low yielding due to side reactions. Typically these reagents and resulting bi-products are harmful to the environment and in the case of Cr(VI) reagents, highly toxic to humans.
Ideally, good atom economy, and low reactivity oxidants should be selected. Hypervalent iodine and heavy metal -based reagents should be avoided
Key references
Heavy metal -based reagents
Ali, M. H.; Leach, D. R.; Schmitz, C. E. A Simple and Efficient Procedure for Oxidation of Sulfides to Sulfoxides on Hydrated Silica Gel with Ceric Ammonium Nitrate (Can) In Methylene Chloride. Syn. Commun. 1998, 28, 2969-2981.
NaIO4/Hypervalent iodine reagents
Johnson, C. R.; Keiser, J. E. METHYL PHENYL SULFOXIDE Org. Synth. 1966, 46, 78.
Relevant scale up examples
Relevant scale up examples – Bleach
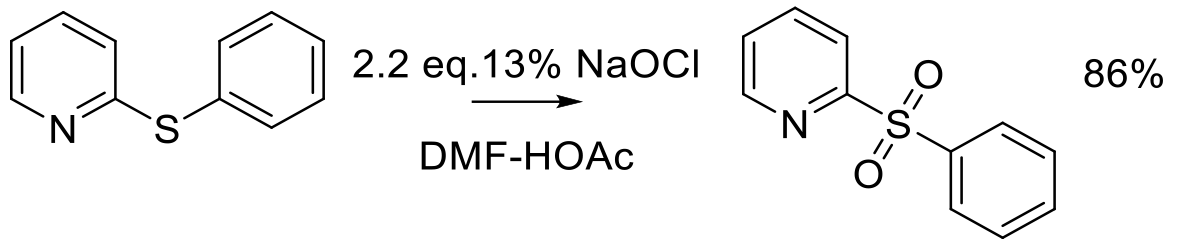
Org. Proc. Res. Dev. 2007, 11, 913-917.
100g scale
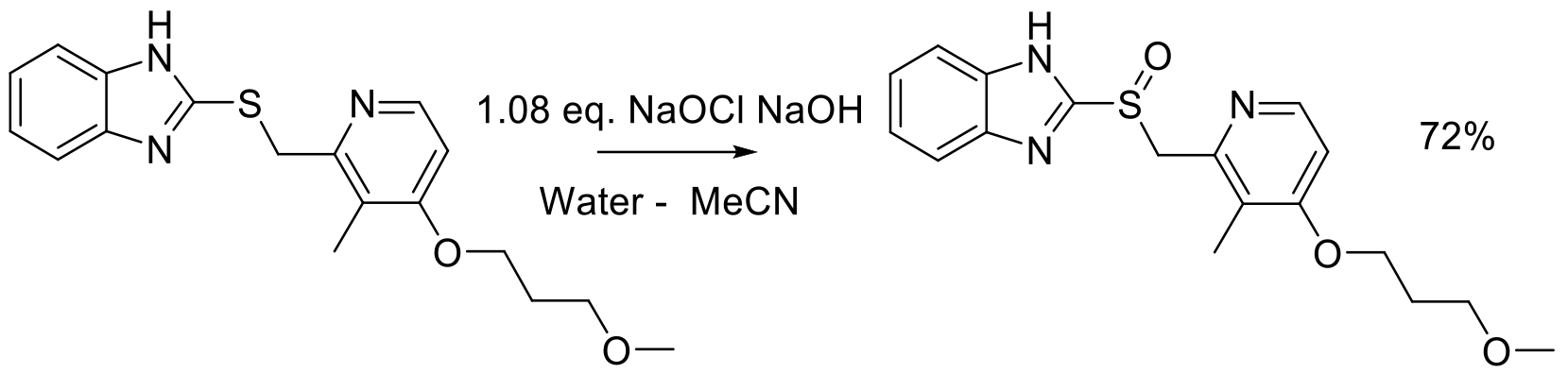
Org. Proc. Res. Dev. 2009, 13, 896-899.
25 kg scale
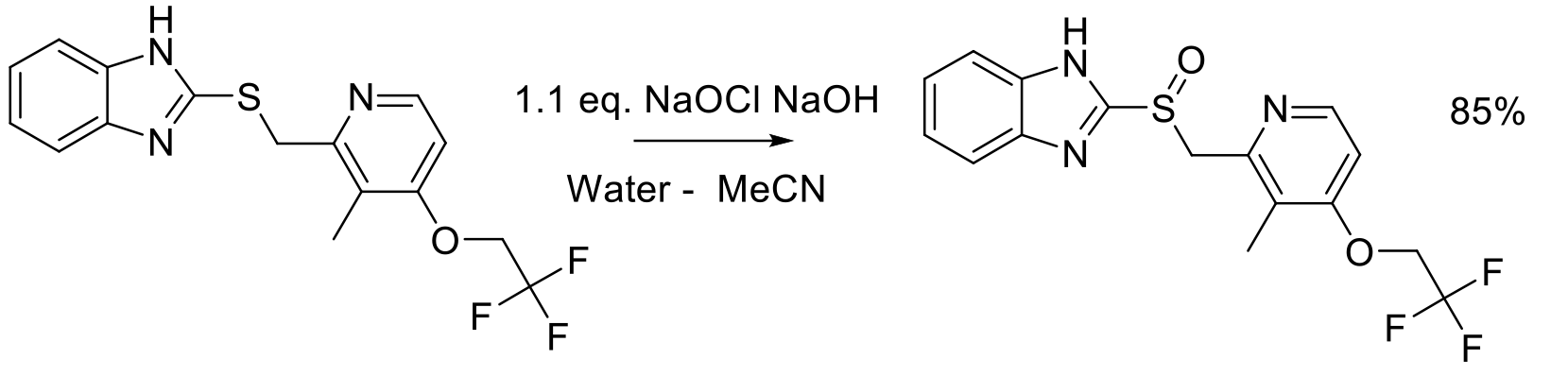
Org. Proc. Res. Dev. 2010, 14, 229-233.
40 kg scale
Relevant scale up examples – Hydrogen peroxide (H2O2)
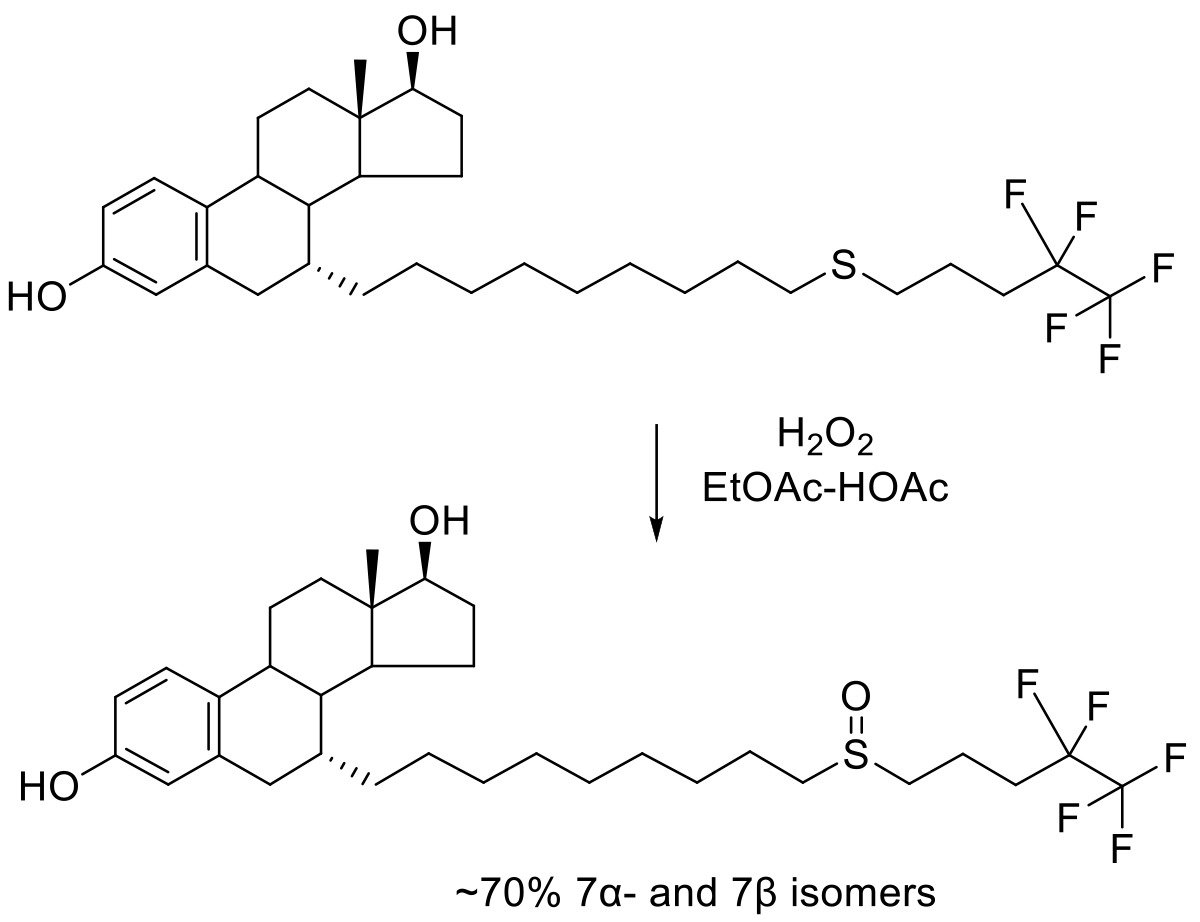
Org. Proc. Res. Dev. 2010, 14, 544–552.
300 kg scale
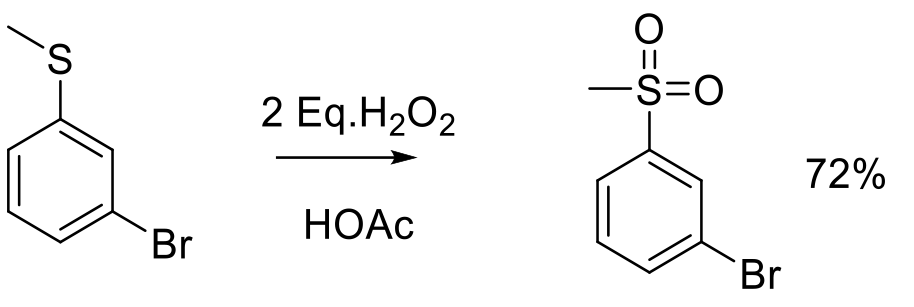
Org. Proc. Res. Dev. 2003, 7, 385-392.
37 kg scale
Relevant scale up examples – Peracids
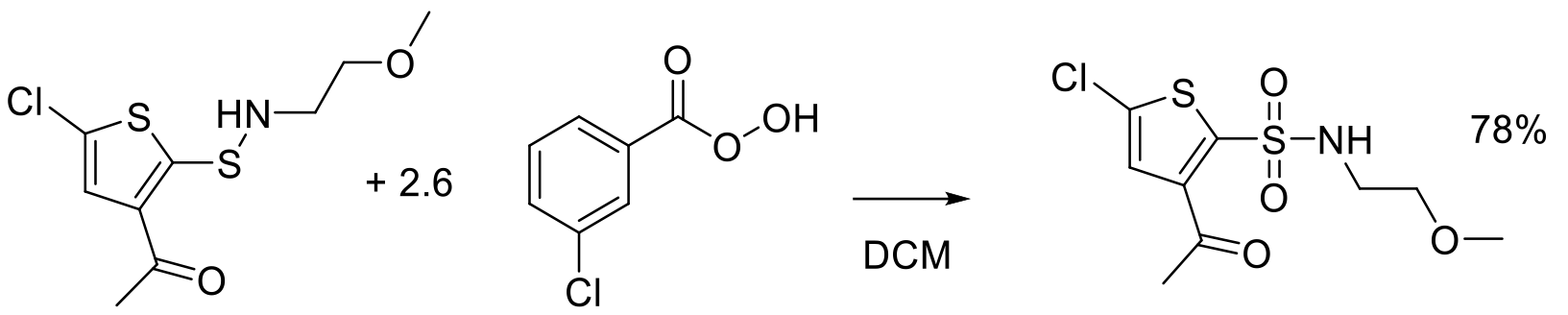
Org. Proc. Res. Dev. 1999, 3, 114-120.
1.5 kg scale
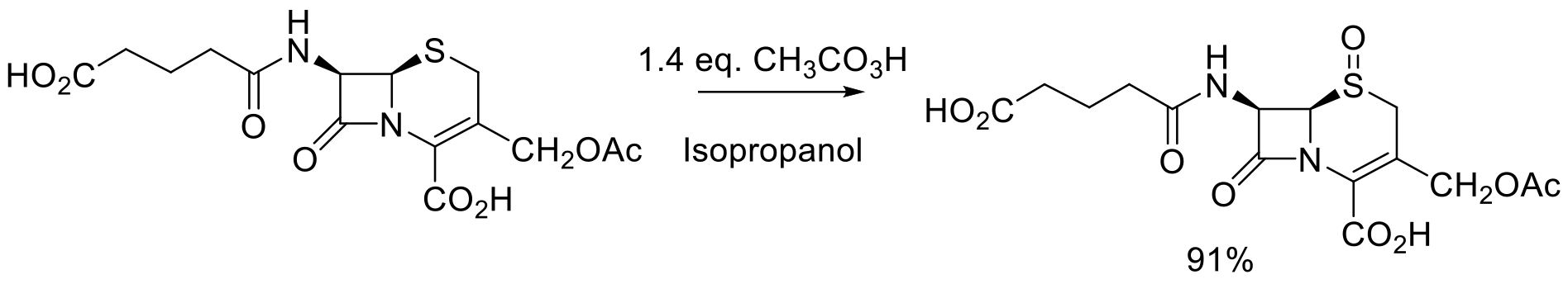
Org. Proc. Res. Dev. 2002, 6, 152-157.
37 kg scale
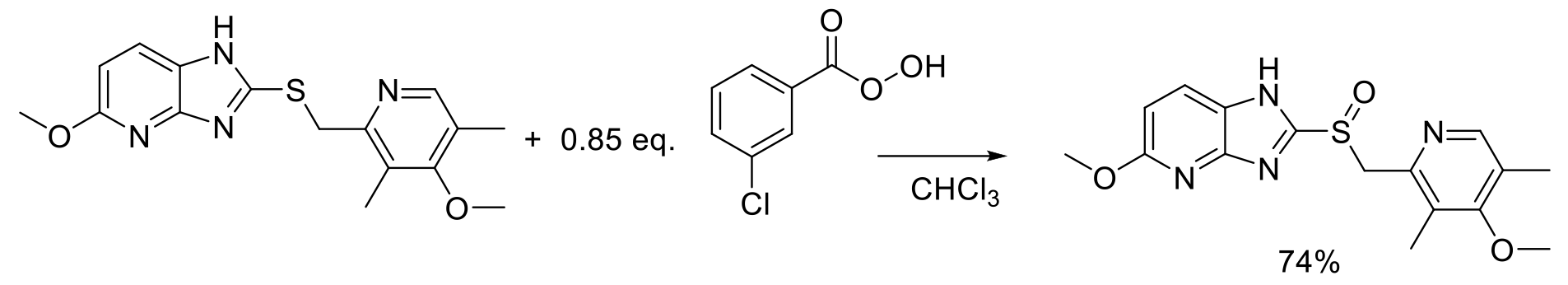
Org. Proc. Res. Dev. 2009, 13, 804-806.
20 kg scale
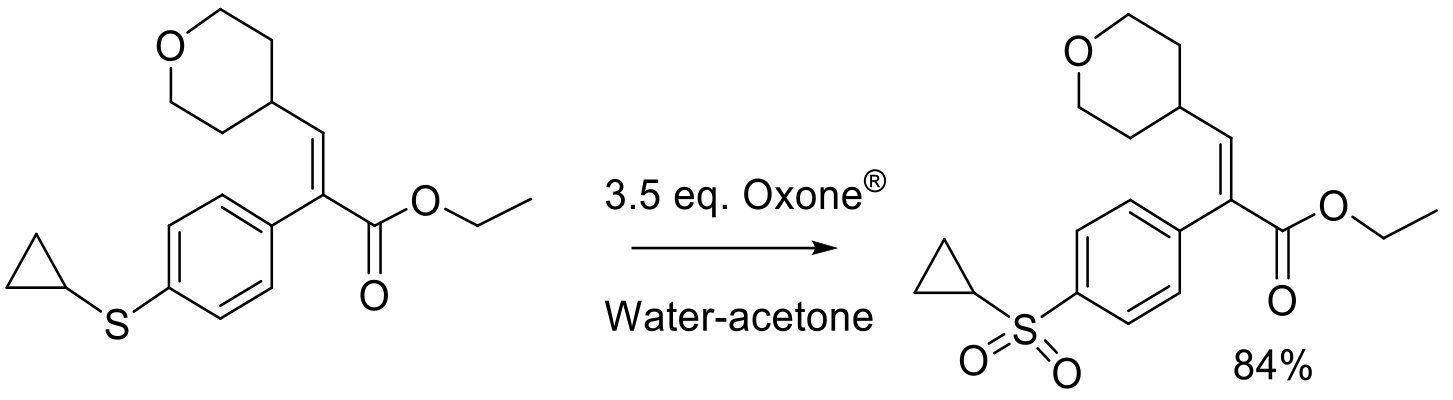
Org. Proc. Res. Dev. 2012, 16, 830-835.
Org. Proc. Res. Dev. 2014, 18, 437-445.
213 kg scale
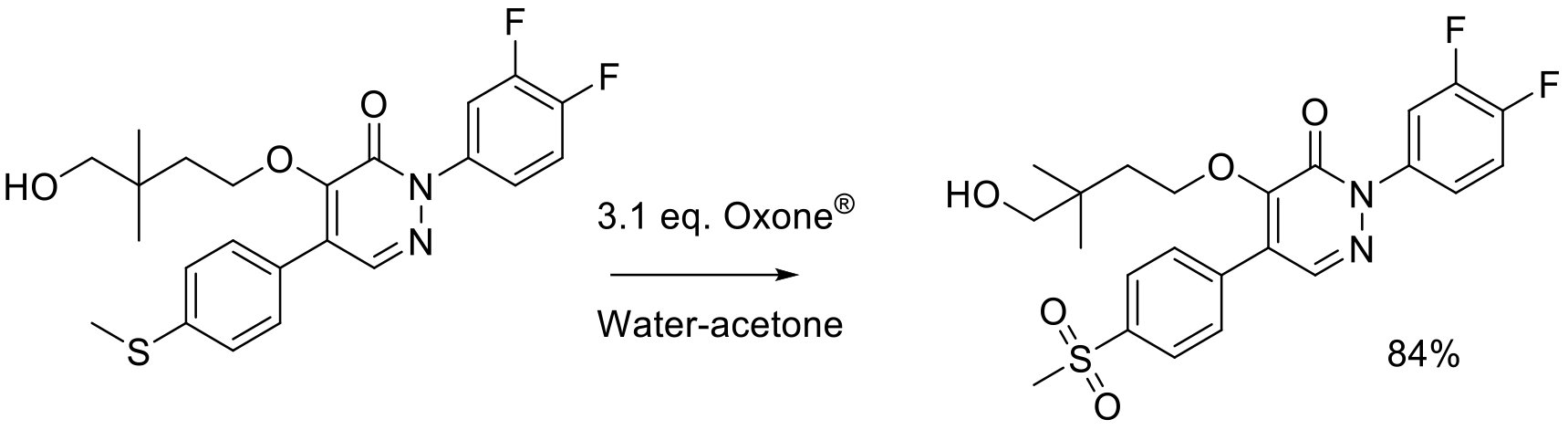
Org. Proc. Res. Dev. 2006, 10, 512-517.
3 kg scale
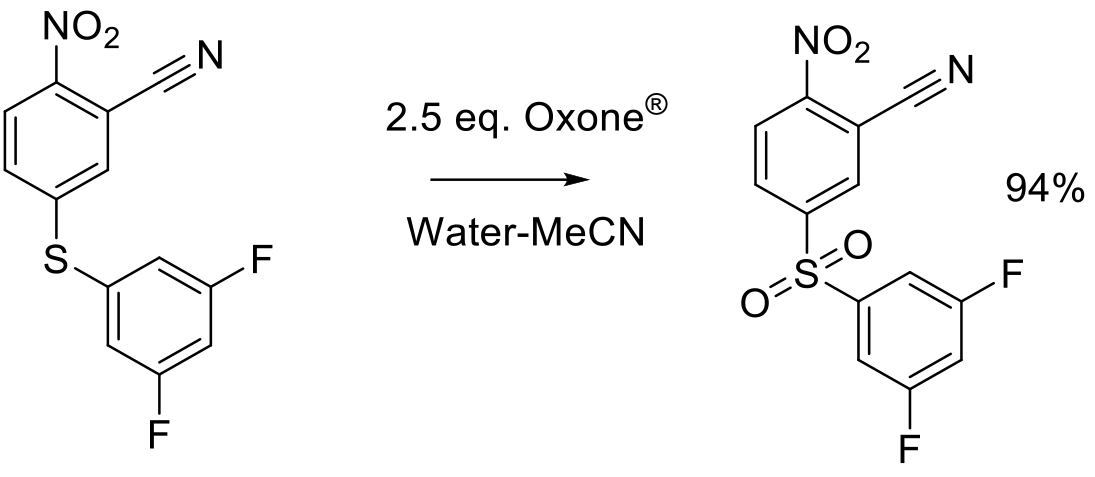
Org. Proc. Res. Dev. 2009, 13, 456-462.
50 g scale
Green Review
-
Atom efficiency (by-products, molecular weight)
Typical terminal oxidants for sulfide oxidation cover a wide range of molecular weights and atom efficiencies. The most attractive for atom economy are O2, H2O2 and NaOCl – other widely used oxidants have poor atom economy, although in some cases -Oxone™/MMPP, the by-products are fairly innocuous. For example:
Oxidant MolWt./ residual MolWt after oxygen transfer
O2 32 and 18 (assume H2O bi-product)
H2O2 34 and 18
NaOCl 74 and 58
MCPBA 172 and 156
Oxone™ 307 and 291
MMPP 494 and 478
- Safety Concerns
Key concerns are safe operation with flammable organic solvents (air/hydrogen peroxide and other reagents that can generate O2), and the potential of oxidizing agents to form unstable mixtures with some reagents/solvents. Oxygen in the presence of air can form an explosive mixture, and measures should be taken to limit the O2 concentration below the limit that would support combustion (see safety section).
Usually hydroperoxides, peroxides and peracids can be explosive when of low molecular weight and in concentrated form. While reagents like Oxone™ and MMPP show poor atom economy, they are reasonably stable and safer to use on scale than reagents with peracetic acid.
No process should be scaled up without appropriate safety testing and concern for exothermic hazards. Before work -up, reactions should be tested and treated with appropriate reducing agents to ensure complete consumption of all oxidizing equivalents – reagent added, or any higher reactivity intermediates formed in situ during the reaction. This should be done before any evaporation, concentration or attempted crystallization of any material from the reaction mixture.
Hypervalent iodine reagents should also be handled with care on scale due potential explosive properties and the potential to generate runaway exothermic reactions.
Mn (VII_ and Chromium (VI) reagents strongly support combustion and can present an explosion hazard in contact with organic materials/solvents. - Toxicity and environmental/aquatic impact
Most higher valent chlorine reagents hydroperoxides, hydroperoxide, peracids -organic and inorganic – (disinfectants) are acutely toxic to most microorganisms. Once reduced, the by-products generally do not present a high degree of concern for the environment.
High concentrations of hypervalent iodine reagents are extremely toxic to aquatic organisms but are probably too reactive to be persistent in the environment. Longer term – Iodinated organics can be persistent and bioaccumulate.
Borate /boron-based reagents will lead to boric acid which is a suspected reprotoxic mutagen.
Traditional heavy metal oxidants like Cr and Mn should be avoided. Chromium (VI) compounds are both acutely toxic (irritant) and known to be carcinogenic to mammals, and are also very hazardous to aquatic organisms. Many Cr (VI) compounds are on the European Union REACH Substances of Very High Concern (SVAC) list due to their carcinogenic properties. Toxic via skin, ingestion and inhalation. - Cost, availability & sustainable feedstocks
Generally no great concerns – most are readily available bulk chemicals and cheap. O2/H2O2/NaOCl are preferred. Hypervalent iodine reagents are generally regarded as expensive for large scale use. - Sustainable implications
Most stoichiometric oxidants used for sulfur oxidation present with few issues. For iodine based reagents, incineration of waste streams could be problematic (iodine content) giving limited utility for waste by-products. Hypervalent iodine reagents are high LCI materials, although it is possible to recover iodide from inorganic and organic waste streams. Iodine is an element at medium to high risk of depletion.
There may be issues with discharging aqueous waste with high boron content. Emerging data suggest boron compounds maybe more ecotoxic than previously thought.
Cr, Ce, Mn reagents often form intractable residues after reaction that can result in protracted cleaning procedures. All metals have a high LCA impact from mining and refining operations, so use should be catalytic with recovery and recycle. Cr and Mn are at risk of limited supply in the future due to depletion.