Catalytic Oxidation
Mechanism + Description
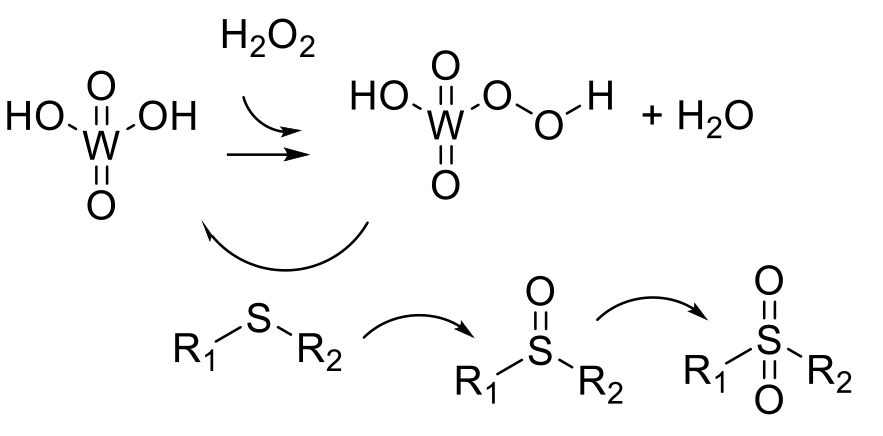
Typically a metal or organic catalyst reacts with an oxidant to give a more reactive species such as a peroxo or hydroperoxide ligated metal center that oxidizes the sulfide substrate to give the sulfoxide or sulfone as required.
General comments
Catalytic oxidation is often employed to enable the utilization of more atom efficient and greener oxidants like H2O2 that otherwise would not react or react slowly with a chosen substrate. This methodology can eliminate the use of poor atom efficiency reagents and the more hazardous oxidants like MCPBA and hypervalent iodine reagents.
Catalysts can be based on metal / metal complexes or organic. A wide range of metals have been used, but those based on non-precious metals (Pt group metals) like W or V based complexes are preferred with H2O2 or O2 as the oxidant. Other reagents such as MCPBA, t-Butyl hydroperoxide and cumene hydroperoxide are also frequently encountered as terminal oxidants.
Organic catalysts or additives are typically ketones or carboxylic acids that will react with the added oxidant to form a more reactive species in situ (peracid or hydroperoxide) which oxidizes the added sulfide substrate.
Key references
Relevant scale up examples
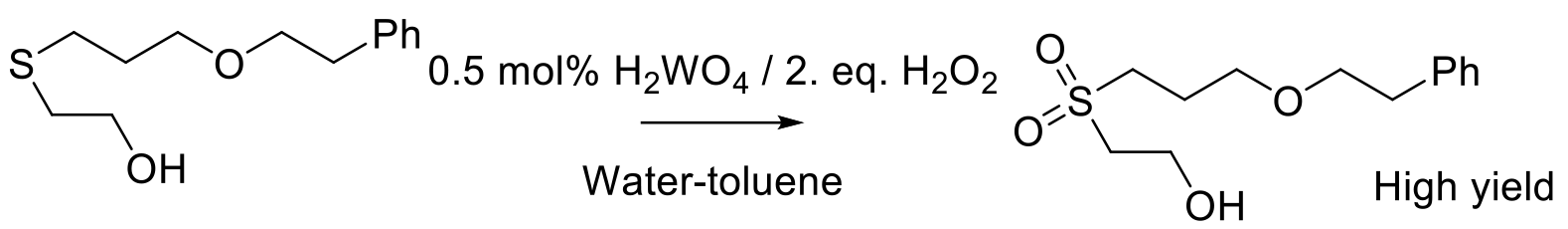
Org. Proc. Res. Dev. 2004, 8, 628-642.
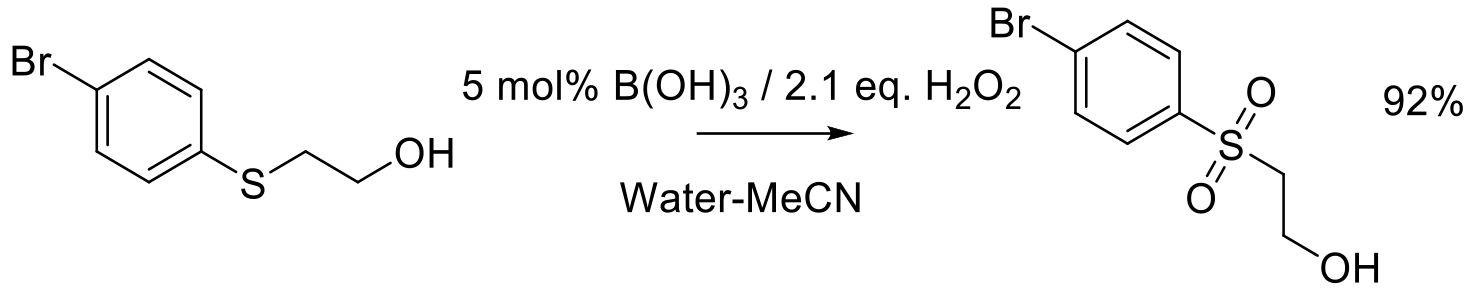
Org. Proc. Res. Dev. 2009, 13, 434-441.
200 g scale
Relevant scale up examples – Halogens/ hypervalent Iodide
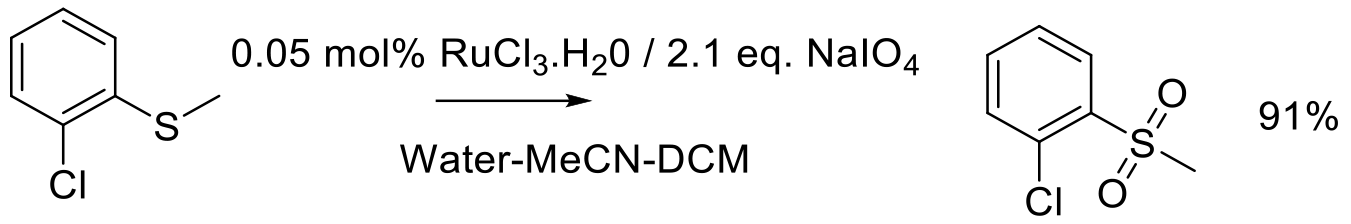
Org. Proc. Res. Dev.2012, 16, 550-555.
3 kg scale
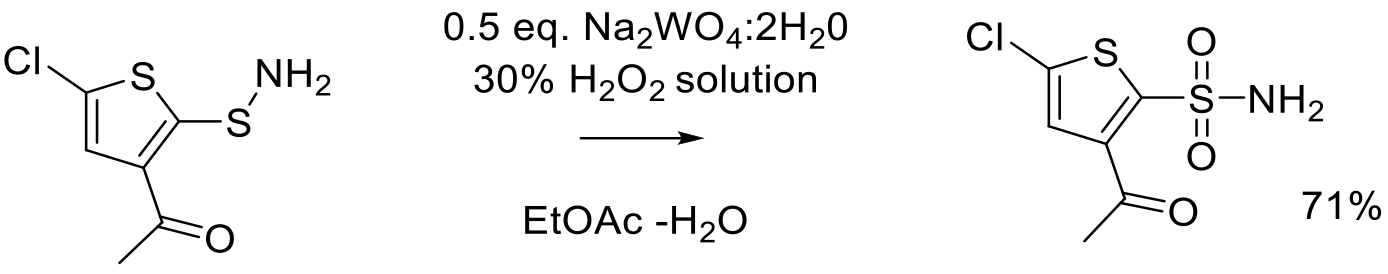
Org. Proc. Res. Dev. 1999, 3, 114-120.
1 kg scale
Green Review
-
Atom efficiency (by-products, molecular weight)
If catalyst charge is optimized (metal plus any ligands) then the bulk of the atom efficiency relates back to the choice of terminal oxidant used (-see pervious section). So low molecular weight reagents giving innocuous by-products are preferred. - Safety Concerns
Some metals/metal complexes are toxic and should be handled with care. Most safety concerns are as for stoichiometric oxidation and avoiding thermal runaways. These have been reported for catalytic sulfur oxidation- see safety section, thus no reaction should be scaled up without appropriate safety testing. Air/O2 -or reagents that could generate O2 in the presence of solvents can generate an explosive mixture – see previous section and safety section. - Toxicity and environmental/aquatic impact
The main concern is around loss of precious metal/ heavy metal catalysts into waste streams. Most precious and heavy metal levels are tightly regulated. The same applies to potential carry through into the API – consult current FDA/ ICH regulations. Some Ni salts are sensitizers and carcinogens and listed on the EU SVAH list. Hydrophobic, high molecular weight ligands such as phosphines can be persistent and bioaccumulative and should not be discharged into aqueous waste streams. - Cost, availability & sustainable feedstocks
With high catalytic efficiency, this methodology can be an economical way to access sulfoxides and sulfones. All metal catalysts have a high LCI from mining and refining and precious metal -based catalysts would need to be recovered and recycled. Generally an abundant metal like W is a good choice. - Sustainable implications
All precious metals have a high life cycle impact (LCI) impact from mining and refining operations, so use should be catalytic with efficient recovery and recycle. Precious metals like Pt, Pd, Ir, and Re are rated at high risk of depletion. No concern for abundant base metals like W, V, Ni, Cu, etc.