Chiral Sulfoxidation/ Biocatalytic Synthesis of Chiral Sulfoxides
Mechanism + Description
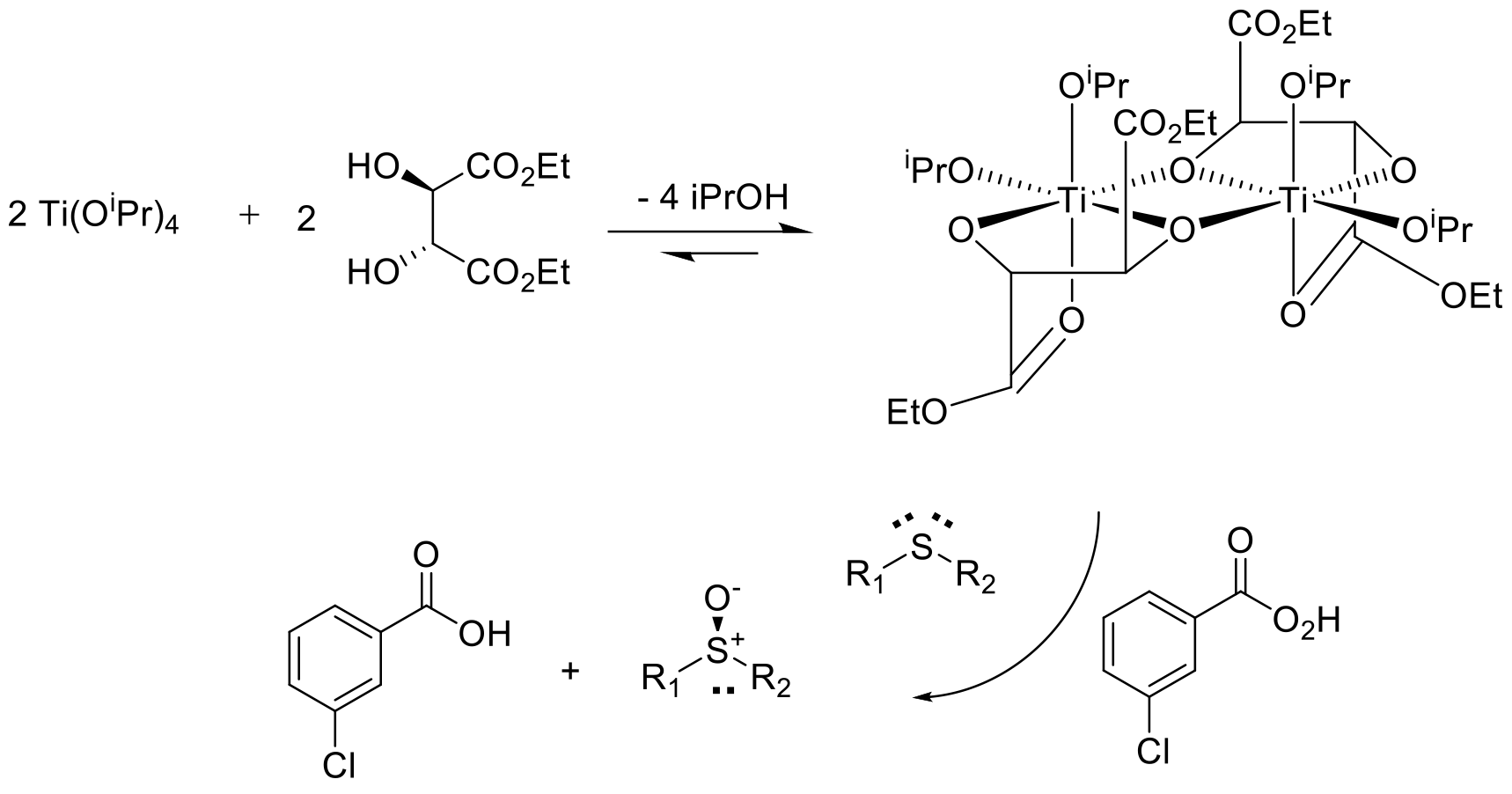
Reaction occurs via a chiral reagent/catalyst that can transfer an oxygen atom to one prochiral face of the R1SR2 sulfide. Typically the sulfide binds to the catalyst / enzyme active site with a terminal oxidant that provides the oxygen atom that can attack one lone pair on the sulfur atom– shown above for a conventional Kagan-type sulfide oxidation. A metal oxo or hydroperoxyl complex can also be involved.
General comments
Chiral sulfoxides are valuable chiral synthons or motifs in final API products. Depending on what other functional groups are also present, the separation of racemic sulfoxide mixtures can be lengthy and resource intensive (chromatography). Hence there is a well-defined requirement for methodologies that directly generate enantiopure sulfoxides from prochiral sulphides (ArSAlkyl, ArSAr1, Alkyl1Salkyl2).
Chiral sulfoxidation can be achieved using stochiometric chiral oxidants or catalytic systems. Stoichiometric oxidants can be based on homochiral organic molecules or metal complexes made with chiral ligands. The Kagan sulfoxidation using Ti(OiPr4) and tartrate ligands is a well-known exemplar.
From a sustainable chemistry aspect, catalytic oxidation based on metal complexes in combination with chiral ligands and a terminal oxidant are preferred. Typical terminal oxidants are inorganic/organic peroxides/hydroperoxides, peracids and air. In most cases, the terminal oxidant is added slowly to the reaction mixture to minimize any oxidation not catalyzed or under control of the chiral agent, which of course would erode the product’s chiral purity. Typical terminal oxidants are inorganic/organic peroxides and peracids.
Biocatalysis can also be used to access chiral sulfoxides, with enzymes like cyp450, peroxidase, Baeyer-Villiger monoperoxygenases (BVMO) producing chiral sulfoxides in high ee. BVMO are preferred enzymes for large scale processes. For this enzyme class, the terminal oxidant is O2. Historically, the use of whole cells and very dilute reactions has hampered productivity, but the application of engineered, heterologously expressed isolated enzymes has addressed this drawback.
Sulfide oxidation has been covered in the biocatalysis reagent guide.
Key references
Relevant scale up examples
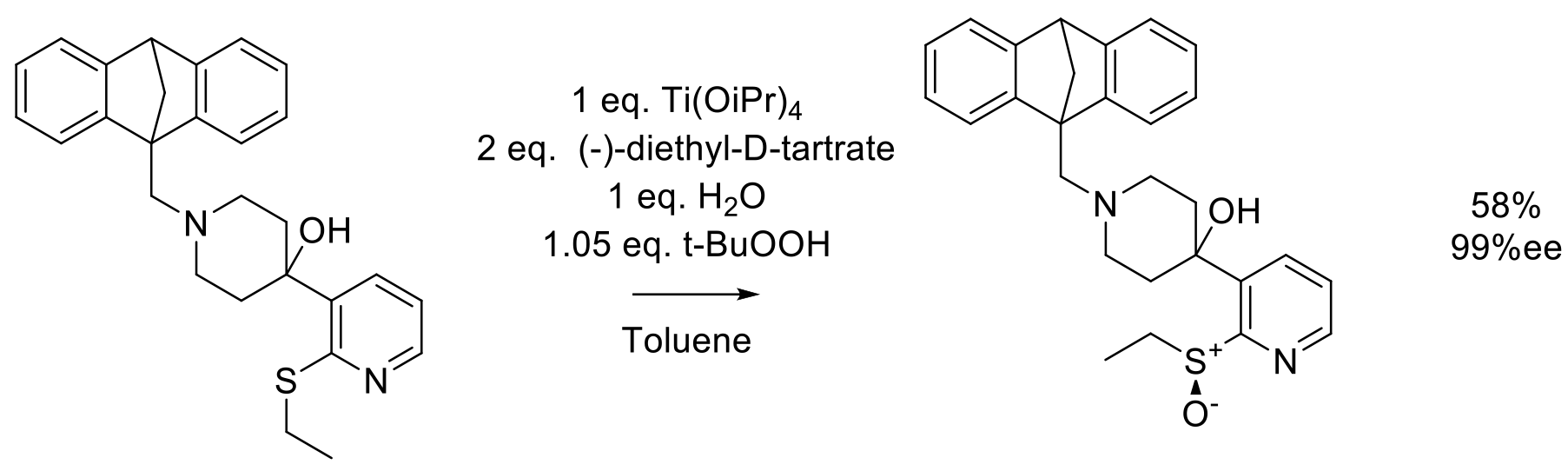
Org. Proc. Res. Dev. 2002, 6, 225-229.
60 g scale
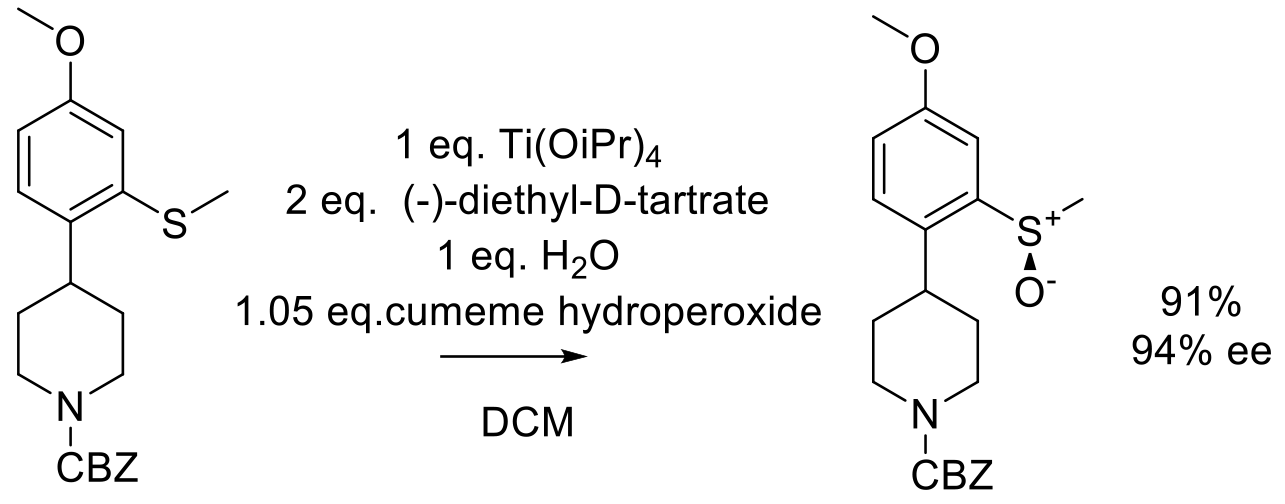
Org. Proc. Res. Dev. 2004, 8, 33-44.
20 g scale
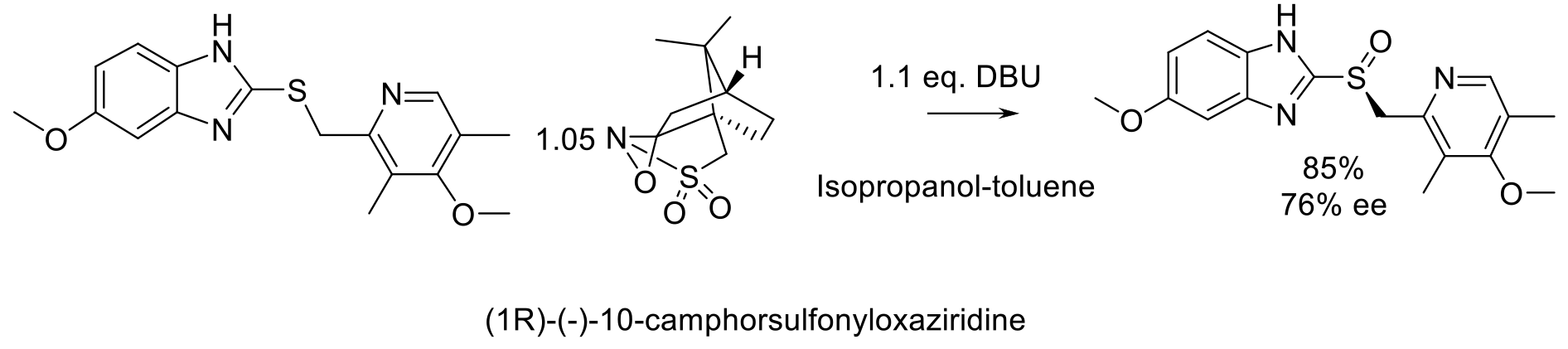
Org. Proc. Res. Dev. 2018, 22, 321-327.
20 kg scale
Relevant scale up examples – Biocatalysis
NB these are updated from the examples shown in the biocatalysis guide
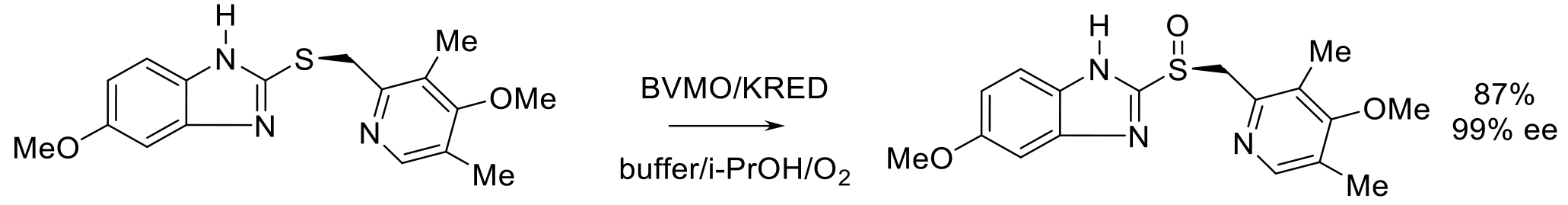
Org. Proc. Res. Dev. 2020, 24, 1124-1130.
J. Org. Chem. 2018, 83, 7453-7458.
1 Kg scale
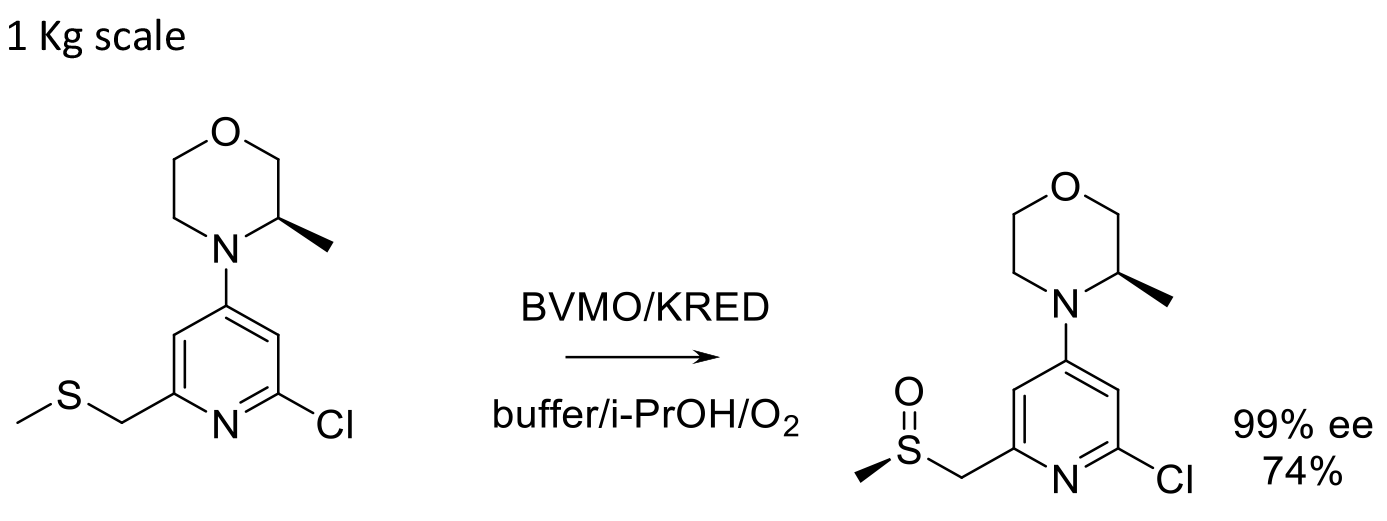
Org. Proc. Res. Dev. 2017, 21, 107-113.
Org. Proc. Res. Dev. 2019, 23, 1333-1342.
90 kg scale
Green Review
-
Atom efficiency (by-products, molecular weight)
If catalyst charge is optimized (metal plus any ligands) then the bulk of the atom efficiency relates back to the choice of terminal oxidant used (-see pervious section). So low molecular weight reagents giving innocuous by-products are preferred. - Safety Concerns
Some metals/metal complexes are toxic and should be handled with care. Most safety concerns are as for stoichiometric oxidation and avoiding thermal runaways. These have been reported for catalytic sulfur oxidation- see safety section, thus no reaction should be scaled up without appropriate safety testing. Air/O2 -or reagents that could generate O2 in the presence of solvents can generate an explosive mixture – see previous section and safety section. - Toxicity and environmental/aquatic impact
The main concern is around loss of precious metal/ heavy metal catalysts into waste streams. Most precious and heavy metal levels are tightly regulated. The same applies to potential carry through into the API – consult current FDA/ ICH regulations. Some Ni salts are sensitizers and carcinogens and listed on the EU SVAH list. Hydrophobic, high molecular weight ligands such as phosphines can be persistent and bioaccumulative and should not be discharged into aqueous waste streams. - Cost, availability & sustainable feedstocks
With high catalytic efficiency, this methodology can be an economical way to access sulfoxides and sulfones. All metal catalysts have a high LCI from mining and refining and precious metal -based catalysts would need to be recovered and recycled. Generally an abundant metal like W is a good choice. - Sustainable implications
All precious metals have a high life cycle impact (LCI) impact from mining and refining operations, so use should be catalytic with efficient recovery and recycle. Precious metals like Pt, Pd, Ir, and Re are rated at high risk of depletion. No concern for abundant base metals like W, V, Ni, Cu, etc.
See also relevant section of the biocatalysis reagent guide.