Oxidation of Thiols to Disulfides/Sulfuryl Chlorides
Mechanism + Description
Reaction occurs via a chiral reagent/catalyst that can transfer an oxygen atom to one prochiral face of the R1SR2 sulfide. Typically the sulfide binds to the catalyst / enzyme active site with a terminal oxidant that provides the oxygen atom that can attack one lone pair on the sulfur atom– shown above for a conventional Kagan-type sulfide oxidation. A metal oxo or hydroperoxyl complex can also be involved.
General comments
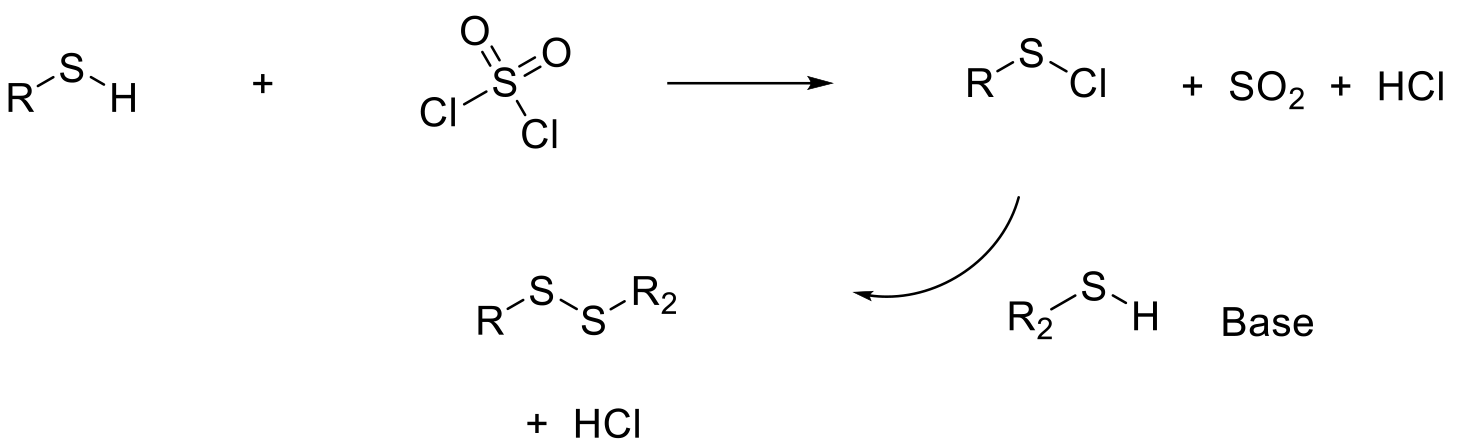
Sulfuryl chlorides are used as intermediates, and such functionality does not appear in final target products. Typically made via oxidation of thiols with halogenating reagents such as Cl2 and SO2Cl2. Sulfuryl chlorides are often used as intermediates to disulfides and are commonly formed and used in situ without isolation. As well as via sulfuryl chlorides, disulfides are commonly made with the oxidative dimerization of thiols in the presence of an oxidant like O2, which proceeds via a radical mechanism.
Key references
Witt, D. Recent Developments in Disulfide Bond Formation Synthesis. 2008, 16, 2491-2509.
Kurzer, F.; Powell, J. R. P-Toluenesulfenyl chloride. Org. Synth. 1955, 35, 99.
Mandala, S.; Basu, B. Recent advances in S–S bond formation RSC Adv., 2014, 4, 13854-13881.
Relevant scale up examples
None found.