Oxidation of Dialkyl Sulphides to Sulfonyl Chlorides
Mechanism + Description
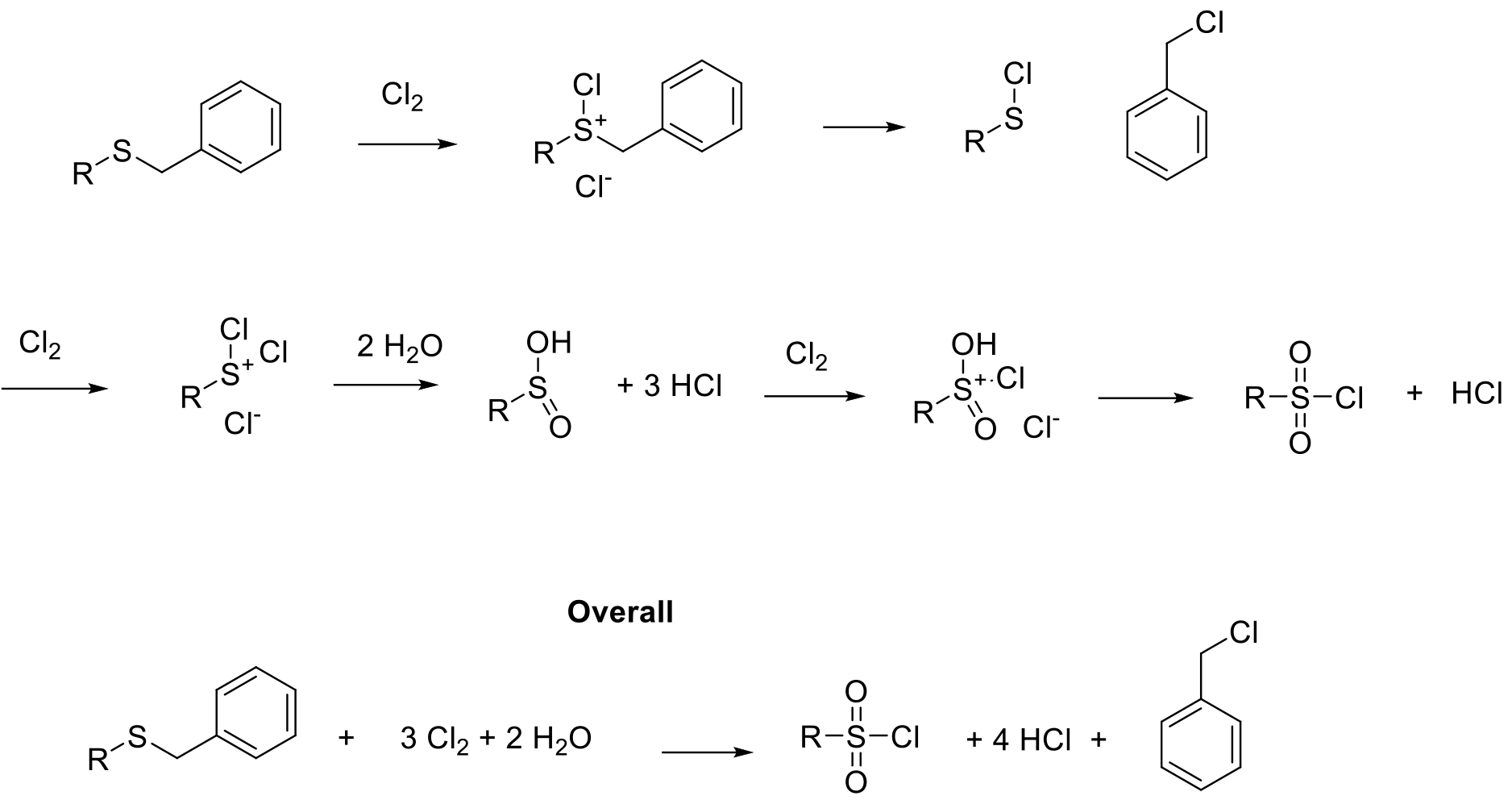
Product is formed by oxidation / cleavage and hydrolysis of cationic chlorinated sulfur species. Stoichiometrically, 3 moles of Cl2 are required.
General comments
Suflonyl chlorides are reactive intermediates, usually used for the synthesis of sulfonamines and sulfonylureas. These are common structural motifs in pharmaceuticals and agrochemicals. Sulfonyl chlorides can be prepared by oxidation of lower valent sulfides and oxidative cleavage of dialkyl sulfides/ alkyl-aryl sulfides. Typical substrates are RSH, RSAc, RSR2. In the case of dialkylsulfides, a preferred leaving group needs to be present to avoid mixtures of products (normally benzyl or t-Butyl).Sulfonyl chlorides can be isolated, but are normally converted on to sulphonamides etc. in one pot type processes. If alkylsulfides are used, a consideration needs to be given to the generation of genotoxic or potential genotoxic (GTI/PGI) by-products such as benzyl chloride / t-BuCl etc.
Key references
www.organic-chemistry.org/synthesis/O2S/sulfonylchlorides.shtm
Relevant scale up examples
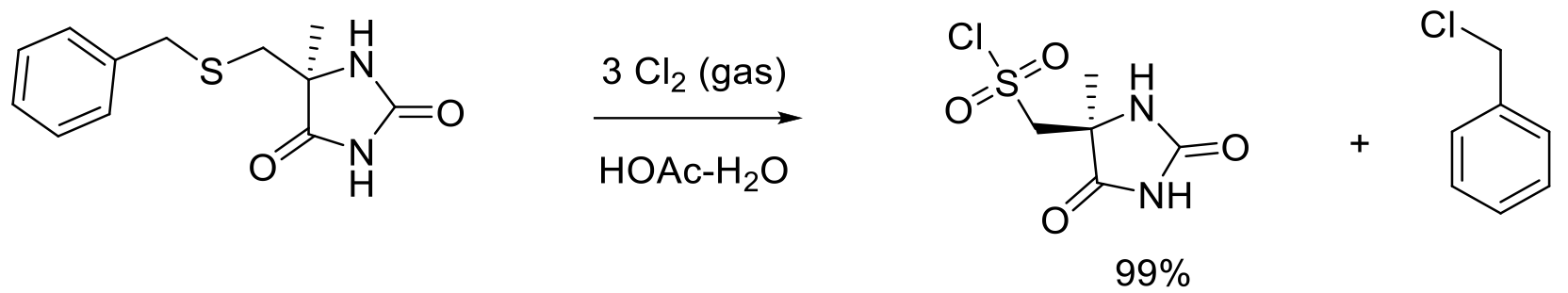
Org. Proc. Res. Dev. 2010, 14, 278-288.
115 kg scale
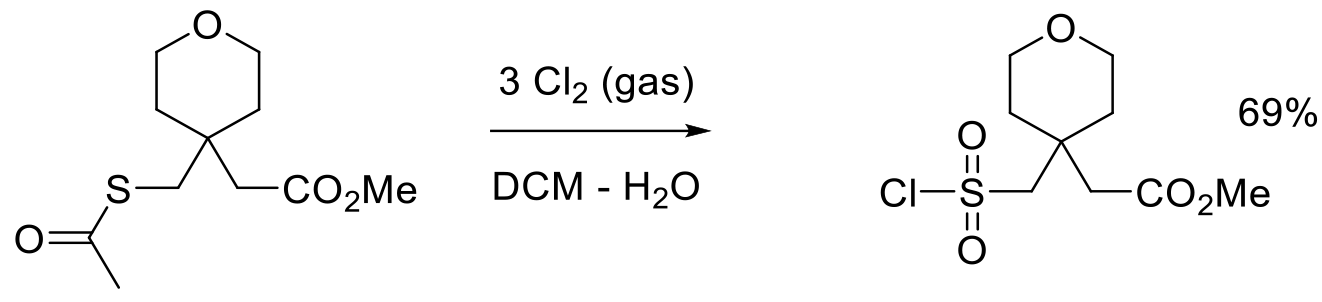
Org. Proc. Res. Dev. 2003, 7, 1034-1039.
4 kg scale