Frustrated Lewis Pairs (FLP)
Mechanism + Description
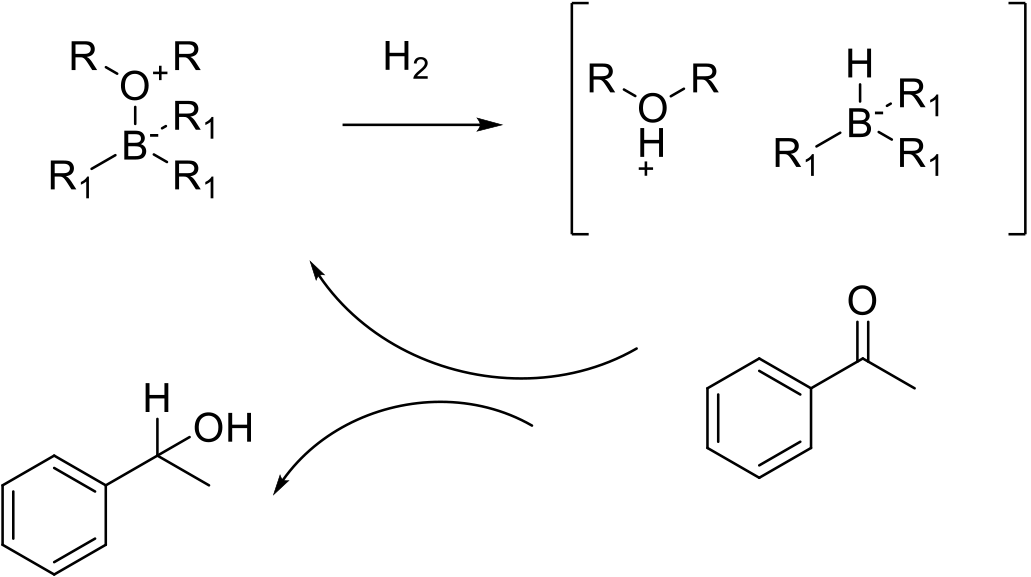
A frustrated Lewis pair (FLP) is a compound or mixture containing a Lewis acidic atom and a Lewis base atom that cannot combine to form a classical donor-acceptor adduct due to steric hindrance. The catalytic cycle involves activation to H2 via bond cleavage to give a protic and hydridic species, so the reduction is stepwise and not concerted as with metal hydrogenations.
General comments
An emerging area providing an organocatalytic route to alcohols from ketones avoiding classical hydride and precious metal catalysts. The FLP catalyst can be added preformed, or made in situ from a Lewis acidic species and a Lewis base-typically a lone pair on a solvent molecule. Chiral FLP catalysts can transfer chirality to the product alcohol by delivering H2 to one prochiral face of the ketone.
Other, older methodologies exist such as Wolff-Kishner Reduction (hydrazine/KOH) / Clemmensen Reduction (Zn/acid), but are rarely encountered these days.
Key references
Stephan, D. W. Frustrated Lewis Pairs: From Concept to Catalysis. Acc. Chem. Res. 2015, 48, 306-316.
Relevant scale up examples
None located.