Mechanism + Description
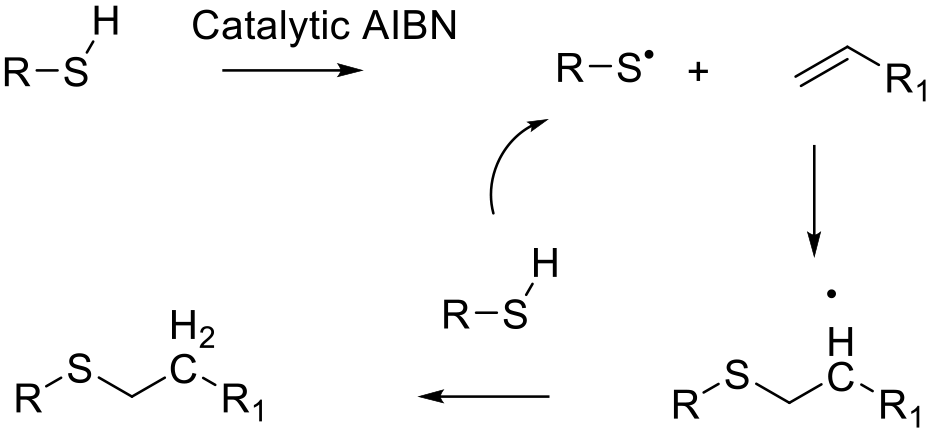
A thiol and radical initiator are used to form thienyl radicals in situ.
These add to the olefin with abstraction of an H radical to form the product and continue the catalytic cycle.
General comments
A possible route to thioethers via alkenes where there is insignificant electro-withdrawing power to effect a conjugate addition. Known as the thiol−ene reaction. Other catalysts can be used instead of traditional radical initiators used in polymer synthesis – metals, photochemistry etc. Addition is usually anti-Markovnikov to give the linear product. As well as linear products, the thiol−ene can be employed as a ring closing reaction to form cyclic thio ethers. A closely related reaction is the addition of thiols to alkynes –thiol-yne reaction generating vinylthioethers. A highly atom-efficient process.
Key references
Sinha, A. K.; Equbal, D. Thiol-Ene Reaction: Synthetic Aspects and Mechanistic Studies of an Anti‐Markovnikov‐Selective Hydrothiolation of Olefins. Asian J. Org. Chem. 2019, 8, 32–47.
Dénès, F.; Pichowicz, M.; Povie, G.; Renaud, P. Thiyl Radical in Organic Synthesis. Chem. Rev. 2014, 114, 2587–2693.
Tang, S.; Liu, K.; Liu, C.; Lei, A. Olefinic C-H functionalization through radical alkenylation. Chem. Soc. Rev. 2015, 44, 1070–1082.
Scanlan, E. M.; Corcé, V.; Malone A. Synthetic Applications of Intramolecular Thiol-Ene "Click" Reactions. Molecules, 2014, 19, 19137–19151.
Ranu, B. C.; Mandal, T. Water-Promoted Highly Selective Anti-Markovnikov Addition of Thiols to Unactivated Alkenes. Synlett, 2007, 925–928.
Dondoni, A.; Marra, A. Metal-Catalyzed and Metal-Free alkyne Hydrothiolation: Synthetic Aspects and Application Trends. Eur. J. Org. Chem. 2014, 3955–3969.
Fadeyi, O. O.; Mousseau, J. J.; Feng, Y.; Allais, C.; Nuhant, P.; Chen, M. Z.; Pierce, B.; Robinson, R. Visible-Light-Driven Photocatalytic Initiation of Radical Thiol-Ene Reactions Using Bismuth Oxide. Org. Lett. 2015, 17, 5756–5759.
Thiol-yne reaction
Tyson, E. L.; Niemeyer, Z. L.; Yoon, T. P. Redox Mediators in Visible Light Photocatalysis: Photocatalytic Radical Thiol-Ene Additions. J. Org. Chem. 2014, 79, 1427–1436.
Xu, B.; Wang, W.; Liu, L.-P.; Han, J.; Jin, Z.; Hammond, G. B. Synthetic evolutions in the nucleophilic addition to alkynes. J. Organomet. Chem. 2010, 696, 269–276.
Hoogenboom, R. Thiol-Yne Chemistry: A Powerful Tool for Creating Highly Functional Materials. Angew. Chem. Int. Ed. 2010, 49, 3415–3417.
Relevant scale up examples

Org. Process Res. Dev. 2004, 8, 628–642.
72 kg scale

