Classical Williamson Type Ether Synthesis: Sulfur as Nucleophile
Mechanism + Description
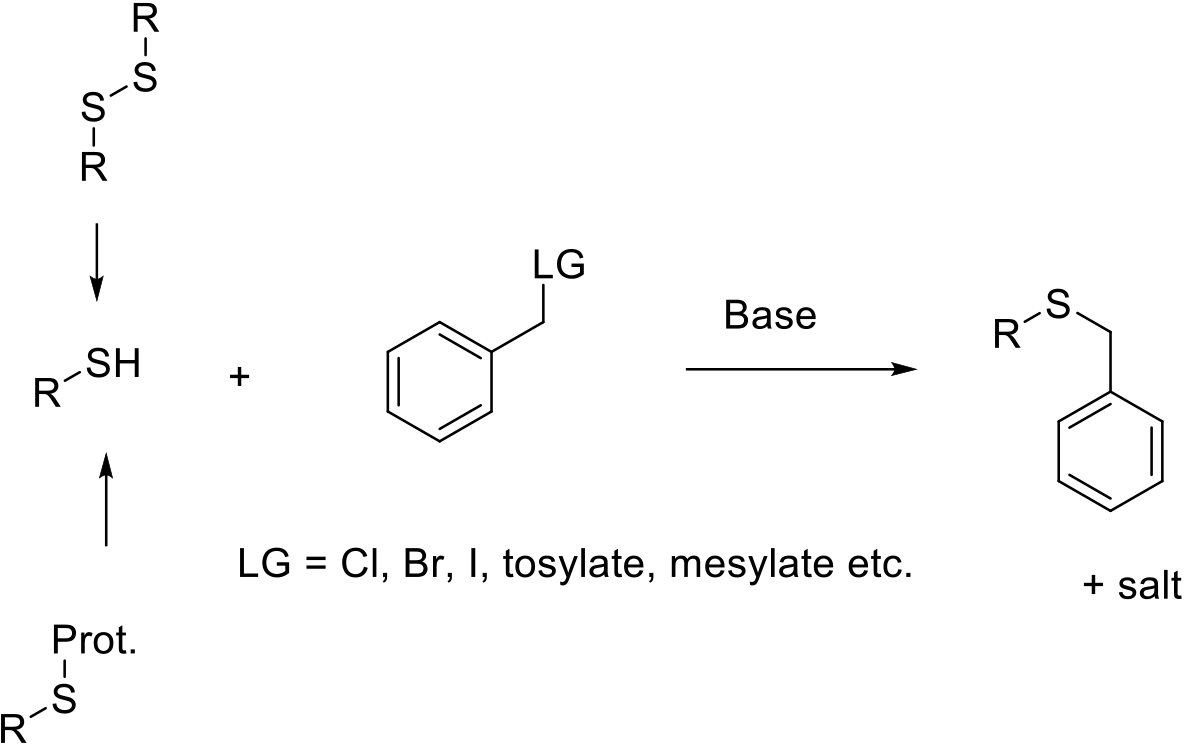
Normally SN2, generating conjugate acid of the leaving group or a salt if a base is added to reduce acidity and reduce protonation of the nucleophile amine. Clean SN2 reaction leads to inversion of a stereocentre at the reacting carbon. Electrophiles capable of forming stable carbocations can react via a SN1 mechanism.
General comments
The construction of carbon–sulfur bonds in the pharmaceutical industry is most widely performed using SN2 or SN1 reactions paralleling the synthesis of amines and ethers. Typically alkylation is achieved by reaction of a thiol or thiolate anion with an activated alkane – SN2 reaction. Typical substrates are chlorides, bromides, iodides, sulfonates, epoxides, and aziridines. With substrates that form stable cations (benzyl, allyl, propargyl), SN1 mechanisms or mixed mechanisms can operate. In SN1 cases, protic or Lewis acids are often added as catalysts. Typically, functionalized alkanes can be prepared and isolated (e.g., halides, sulfonates), although in some cases, electrophilic partners are prepared in situ. Mitsunobu alkylation is widely used on lab scale and has been used at large scale, although separation of by-products like Ph3PO can be an issue.
Typically thioether preparation is via reaction with the thiol, alkylating agent and a base in a dipolar aprotic solvent. Many of the traditional dipolar aprotic solvents like DMF, NMP, DMAC are reprotoxic and should be avoided if possible.
As with the Williamson ether synthesis, SN2 synthesis of thioethers can often be beneficially run under phase transfer conditions (PTC). See N-alkylation at sp3 carbon reagent guide for further analysis of PTC reaction conditions.
Many alkylating agents are mutagens or suspected mutagens, i.e., give positive alerts for genotoxicity. Hence if alkyl halides/sulfonates are to be used, the least reactive leaving group commensurate with desired reactivity should be selected, excess reagent avoided, and the reaction placed as early in the synthetic sequence as feasible. Other electrophiles like epoxides and aziridines give functionalized thioethers but also suffer from giving positive alerts for genotoxicity.
Key references
Eccles, K. S.; Elcoate, C. J.; Lawrence, S. E.; Maguire, A. R. Convenient and robust one-pot synthesis of symmetrical and unsymmetrical benzyl thioethers from benzyl halides using thiourea ARKIVOC, 2010, (ix), 216–228.
Soleiman-Beigi, M.; Kazemi, M.; Aryan, R.; Shiri, L. TBAOH Mediated: An Efficient and Simple Procedure for Alkylation of Alcohols, Phenols and Thiols Under Neat Aqueous Conditions Letters in Organic Chemistry, 2014, 11, 321–326.
Relevant scale up examples

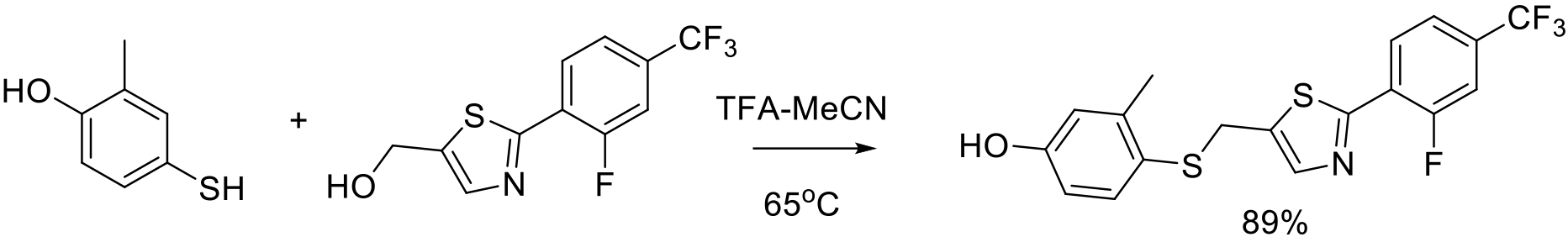
Org. Process. Res. Dev. 2003, 7, 839–845.
100 g scale
Org. Process Res. Dev. 2009, 13, 297–302.
50 kg scale
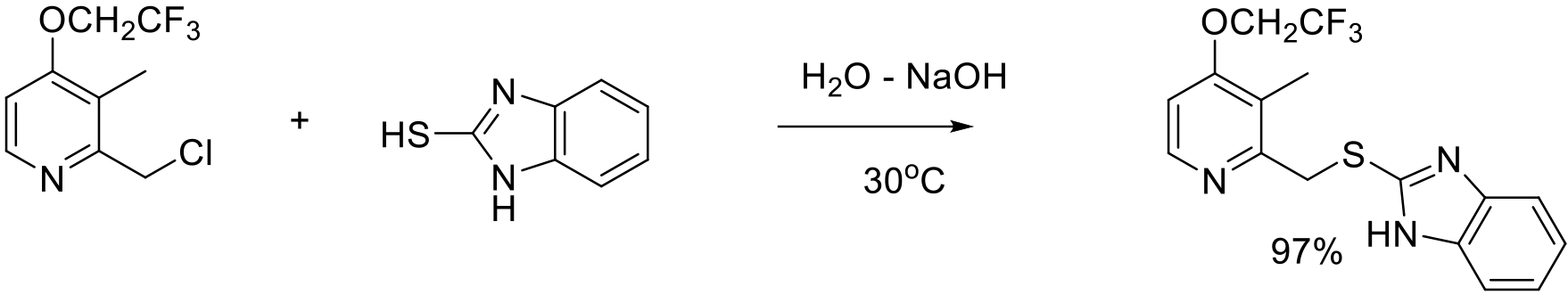
Org. Process Res. Dev. 2010, 14, 229–233.
50 kg scale

Org. Process Res. Dev. 2010, 14, 544–552.
120 kg scale

Org. Process Res. Dev. 2011, 15, 855–857.
3 kg scale
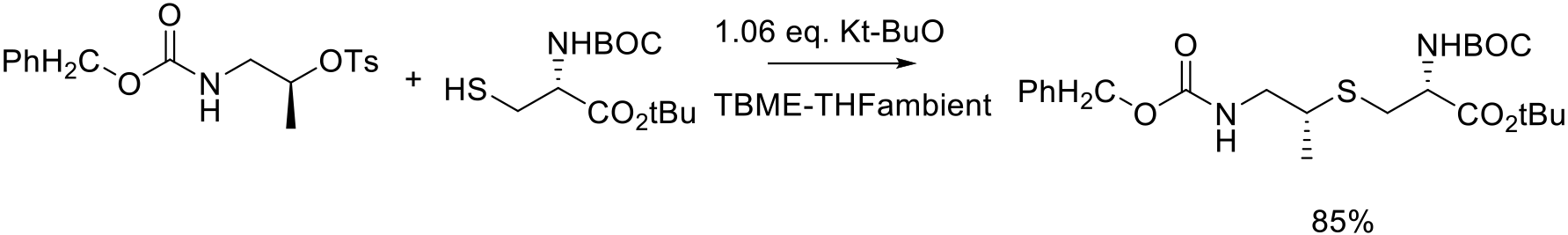
Org. Process Res. Dev. 2009, 13, 774–780.
75 kg scale
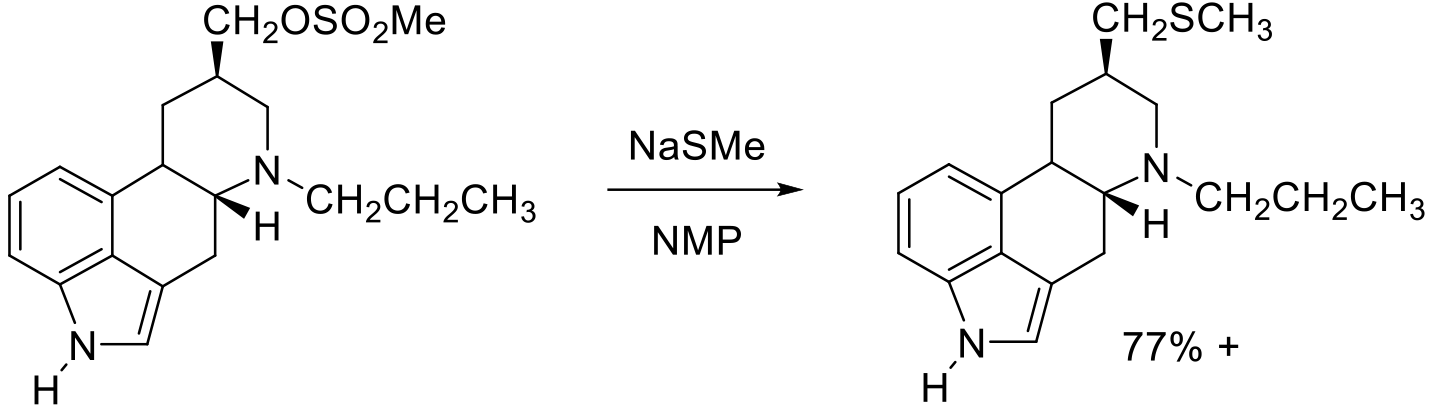
Org. Process Res. Dev. 2006, 10, 198–202.
10 kg scale

Org. Process Res. Dev. 2010, 14, 425–431.
1 kg scale
Green Review
-
Atom efficiency (by-products, molecular weight)
This comes down to the leaving group Cl <Br <I. Sulfates and sulfonic esters produce high or higher mol. wt. by-products than Cl or Br. Low mol. wt. leaving groups are preferred. Mitsunobu alkylations have poor atom efficiency. - Safety Concerns
No major safety concerns with the operational aspects of alkylation. All alkylating agents will have positive PGI alerts and will be known or suspected carcinogens, so should be handled with appropriate caution. Use of alkylating agents towards the end of a synthesis may give a requirement to measure very low levels of alkylating agent in the API. See Org. Process Res. Dev. 2013, 17, 221−230. The level of concern rises with reactivity of the electrophile. Low mol. wt. sulfonates can also be highly acutely toxic, e.g., methyl fluorosulfonate (‘magic methyl’). H2S or volatile low mol. wt. alkyl thiols like MeSH are highly toxic. - Toxicity and environmental/aquatic impact
This is really down to the ability of the reagent to biodegrade and its reactivity. Some are very toxic, e.g., benzyl halides. Higher molecular weight, lipophilic materials may show bioaccumulation. Sulfates and sulfonate esters are generally rapidly hydrolyzed and environmental concerns reflect those of the alcohol. Fluorosulfonated anions can be persistent in the environment. High concentrations of iodide is harmful to freshwater ecosystems.
The use of reprotoxic dipolar aprotic solvents should be avoided if possible. Lower mol. wt. alkylsulfides, thioethers and H2S are highly malodorous and reaction off-gases should be scrubbed with an oxidant like NaOCl. - Cost, availability & sustainable feedstocks
Many alkyl halides are readily available and cheap or can be prepared from the corresponding alcohols. Sulfates and sulfonate esters can be prepared in situ just before use to minimize handling and the potential of exposure. - Sustainable implications
Incineration of waste streams could be problematic (iodine content). Limited utility for waste by-products. Iodine is an element at medium to high risk of depletion. It is a high LCI reagent, although it is possible to recover iodide from organoiodine waste materials. Li and Cs bases should be avoided if possible.