Reaction of Arenes/Heteroarenes with Thiols – SNAr Chemistry
Mechanism + Description
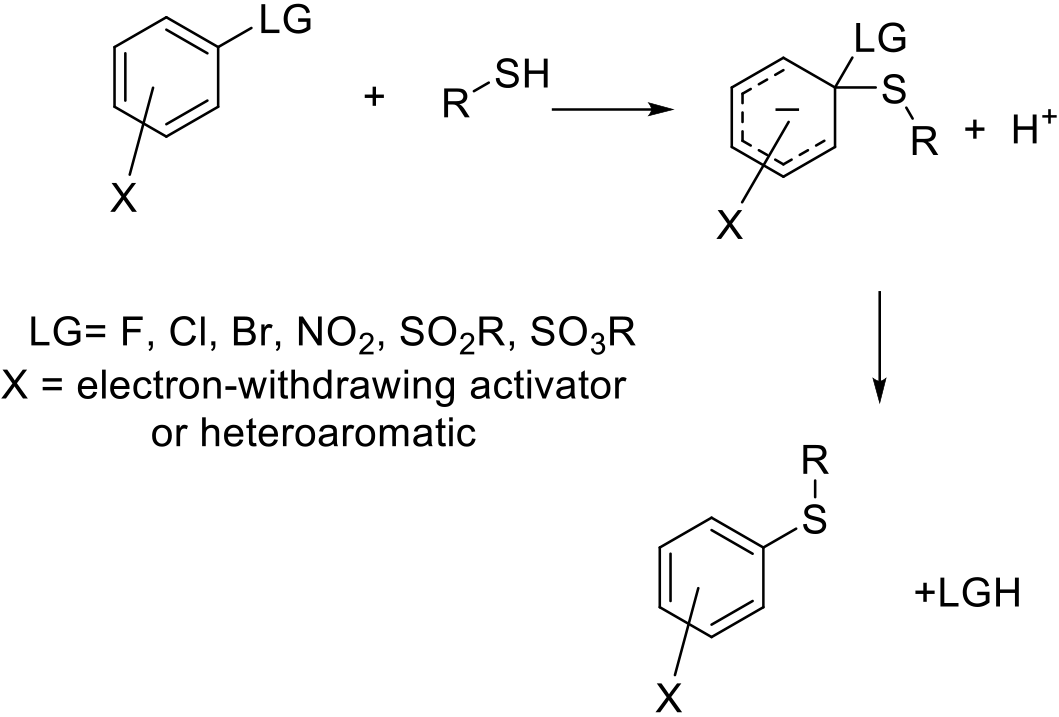
The SNAr involves nucleophilic displacement of a leaving group by a nucleophile—usually RSH or RS- salt. Typically, the arene is activated to substitution by the presence of electron-withdrawing groups or is an electron-deficient heteroarene. The nucleophile attacks the ipso carbon to form an intermediate Meisenheimer complex. Loss of an acid or salt then generates the product.
General comments
Typically, aryl fluorides and heteroaryl chlorides are the substrates of choice for the SNAr reaction, although other leaving groups like bromide, sulfonate or nitrate are sometimes used. The reaction is typically performed by reaction of the aryl/heteroaryl halide with the thiol in a solvent in the presence of a base. Typically, SNAr reactions are intermolecular between an electrophile and a nucleophile, but can be used in an intramolecular sense for the construction of fused heterocycles. If the nucleophile is weak, or for particularly unreactive aryl halides, prior deprotonation of the thiol partner with a strong base is used. Bases are typically tert-amines, like Et3N or Hünig’s base; inorganics like Na2CO3, K2CO3, KOH, NaOH, Cs2CO3; or, stronger bases, like NaH, Kt-BuO, LHMDS, etc. The use of NaH/KH with dipolar aprotic solvents is not recommended on safety grounds—see solvent section for more details.
Typical dipolar aprotic solvents used for SNAr chemistry are DMF, DMAC, NMP. These have been identified as being reprotoxic (H360) and should be replaced if possible. Less widely utilised dipolar aprotic solvents like N-ethylpyrrolidinone (NEP), tetramethyl urea (TMU), 1,3-Dimethyl-3,4,5,6-tetrahydro-2-pyrimidinone (DMPU) and 1,3-Dimethyl-2-imidazolidinone (DMI) have been put forward as replacements for DMF/NMP/DMAC, but recent data indicates that these solvents are also reprotoxic.
Some cyclic amides like N-Butylpyrrolidinone (NBP) have been put forward as non-reprotoxic alternatives for a range of chemical transformations. Available data suggests that higher molecular weight, more lipophilic cyclic amides like NBP do not show the adverse reprotoxic effects shown by the lower, hydrophilic dipolar aprotic solvents. MeCN has a good toxicity profile, but is expensive and has suffered from supply chain issues in the past.
Key references
Relevant scale up examples

Org. Process Res. Dev. 2012, 16, 697−703.
1 kg scale

Org. Process Res. Dev. 2007, 11, 913–917.
50 g scale
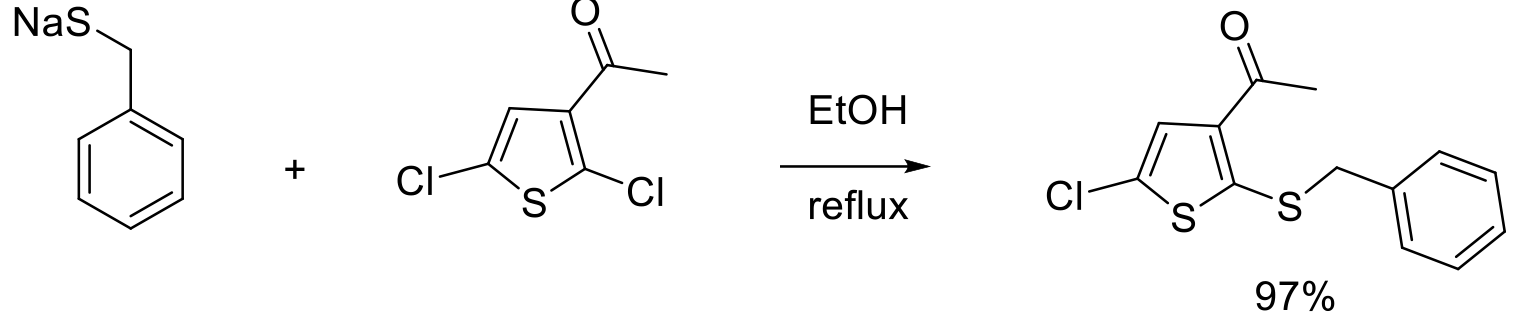
Org. Process Res. Dev. 1999, 3, 114–120.
1 kg scale
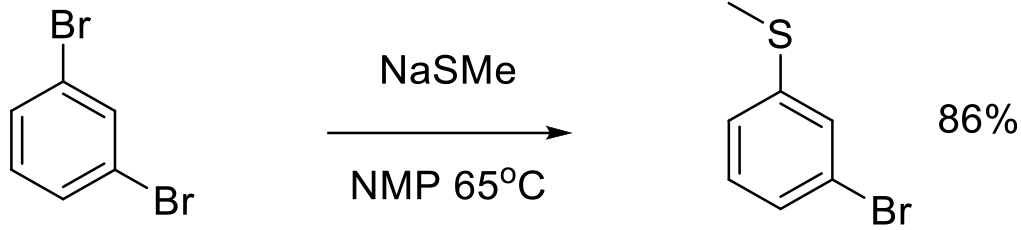
Org. Process. Res. Dev. 2003, 7, 385–392.
40 kg scale
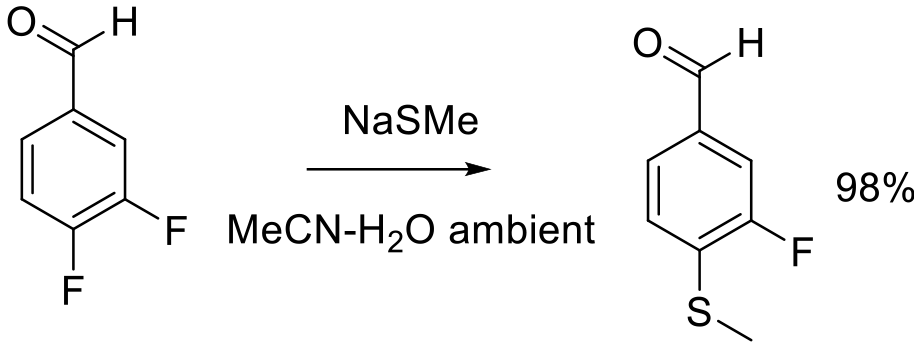
Org. Process. Res. Dev. 2008, 12, 111–115.
30 g scale
Green Review
-
Atom efficiency (by-products, molecular weight)
This comes down to the leaving group—by-products being conjugate salts / acids. F < Cl < Br < I. Sulfates and sulfonic esters produce high or higher mol. wt. by-products. - Safety Concerns
No major safety concerns with the operational aspects of SNAr chemistry. H2S or volatile low mol. wt. alkyl thiols like MeSH are highly toxic. - Toxicity and environmental/aquatic impact
This is really down to the ability of the reagents to biodegrade and their reactivity. Some are very toxic, e.g., benzyl halides. Higher molecular weight, lipophilic materials may show bioaccumulation. Fluorinated lipophilic aromatics can be persistent in the environment. High concentrations of iodide is harmful to freshwater ecosystems.
The use of reprotoxic dipolar aprotic solvents should be avoided if possible.
Lower mol. wt. alkyl sulfides, thioethers and H2S are highly malodorous and reaction off-gases should be scrubbed with an oxidant like NaOCl. - Cost, availability & sustainable feedstocks
Most are readily available from petrochemical feedstocks. - Sustainable implications
Incineration of waste streams could be problematic (iodine content). Limited utility for waste by-products. Iodine is an element at medium to high risk of depletion. A high LCI reagent, although it is possible to recover iodide from organoiodine waste materials. Li and Cs bases should be avoided if possible.