Cyclopropanation Catalysed by p450enzymes/hemes
Mechanism + Description
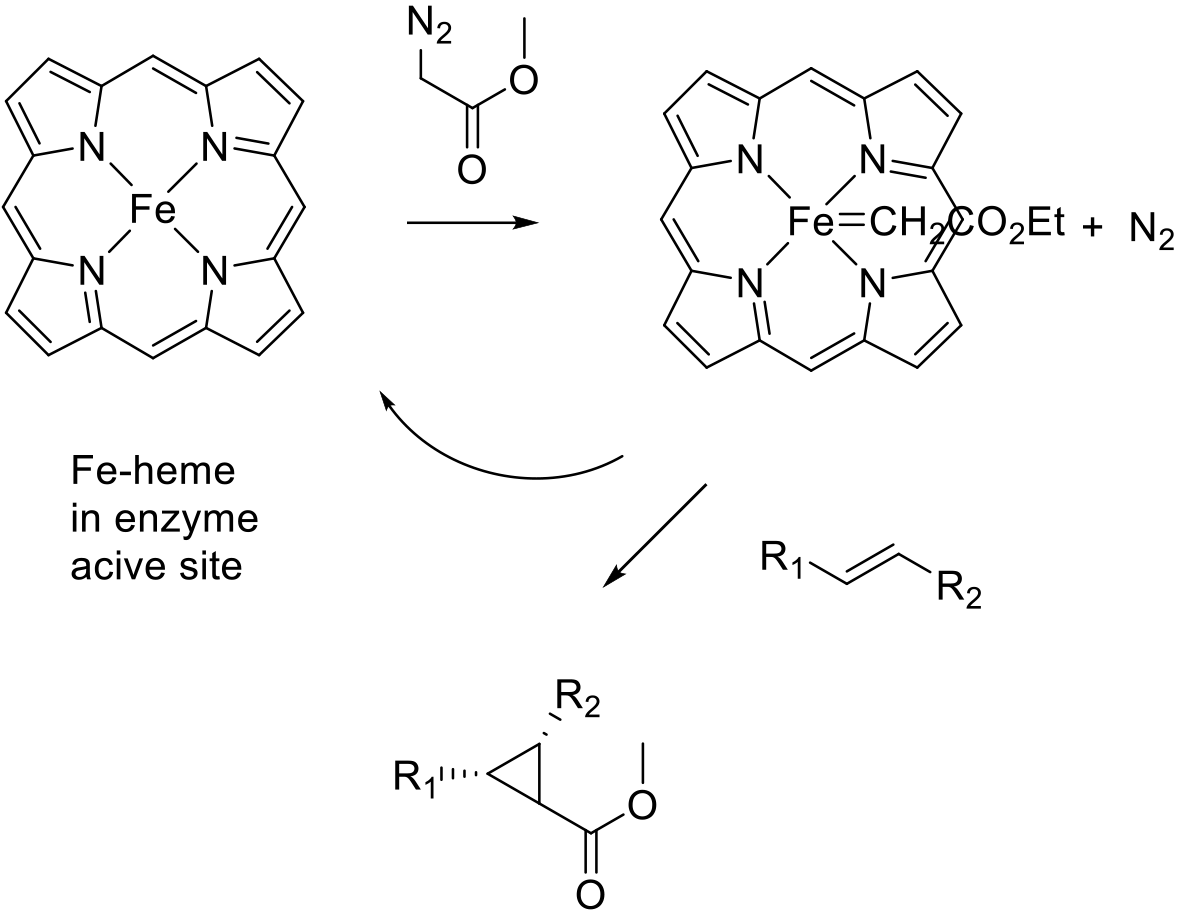
The mechanism closely follows that of cyclopropanation by chiral metal-carbene complexes. Diazoalkanes are used to form a metal carbene intermediate on the iron atom of a heme complex plus nitrogen. Since the metal-carbene complex sits in a highly chiral environment in the active site of the enzyme, transfer of the carbene occurs to one prochiral face of an alkene substrate generating chiral cyclopropane products.
General comments
The use of iron-heme containing enzymes to make chiral cyclopropanes is an interesting, and potentially, a very useful example of promiscuous enzyme catalysis. Since the reaction is not a classical p450 transformation like hydroxylation, the complex and sensitive electron transport mechanisms used to complete the catalytic cycle in p450 hydroxylation are not needed, making the scale-up of p450-catalysed cyclopropanation potentially much easier. This reaction was first noted with p450 enzymes but has since been extended to other Fe containing enzymes and iron–heme proteins. Key attractions of this methodology are the avoidance of precious metal-based catalysts, and the products are chiral. As opposed to say synthetic phosphine/nitrogen ligands, rapid directed evolution techniques can be used to make many 100’s or 1000’s of new variants to accept new substrates and optimise the e.e. of the resulting products.
Key references
Relevant scale up examples
None located.