Kulinkovich Cyclopropanation
Mechanism + Description
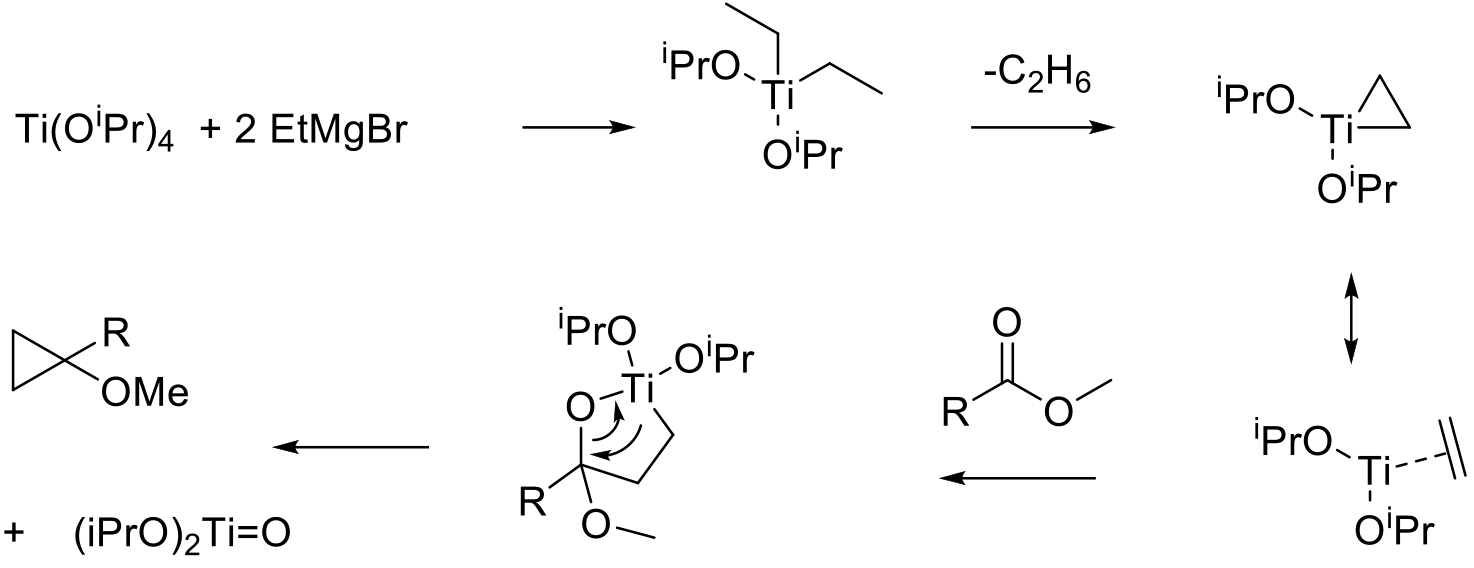
Reaction of a Ti(IV) alkoxide with 2 eq. of an organometallic gives a reactive Ti –carbene like intermediate after elimination of an alkane. This reactive species adds to the C=O giving a titanocycle. This rearranges to give the product following elimination of a Ti-Oxo species.
General comments
A method used to prepare substituted cyclopropanes. The reaction uses a stoichiometric amount of titanium(IV) isopropoxide (or methyltitanium triisopropoxide) and an ethyl organometallic. The initial adduct breaks down releasing ethane to give the reactive Ti organometallic intermediate. This has nucleophilic character and adds to the C=O bond of the substrate giving the product and a Ti oxo by-product. The driving force of the reaction being the formation of the strong Ti=O bond. Originally developed with esters, a number of related variants exist, e.g., aza-Kulinkovich or the Kulinkovich-de Meijere, Kulinkovich− Szymoniak cyclopropanation reactions using substrates such as amides and nitriles to give cyclopropylamines as products.
Key references
Relevant scale up examples
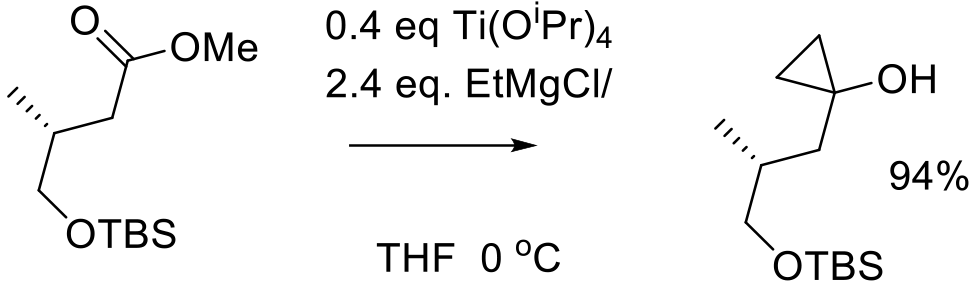
Org. Process Res. Dev. 2015, 19, 1360−1368.
44 kg scale
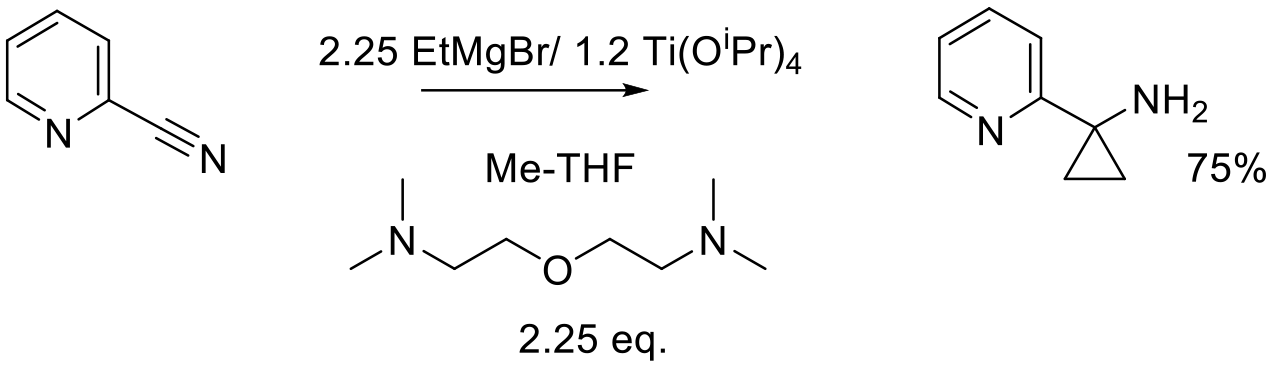
Org. Process Res. Dev. 2012, 16, 836−839.
8 kg scale
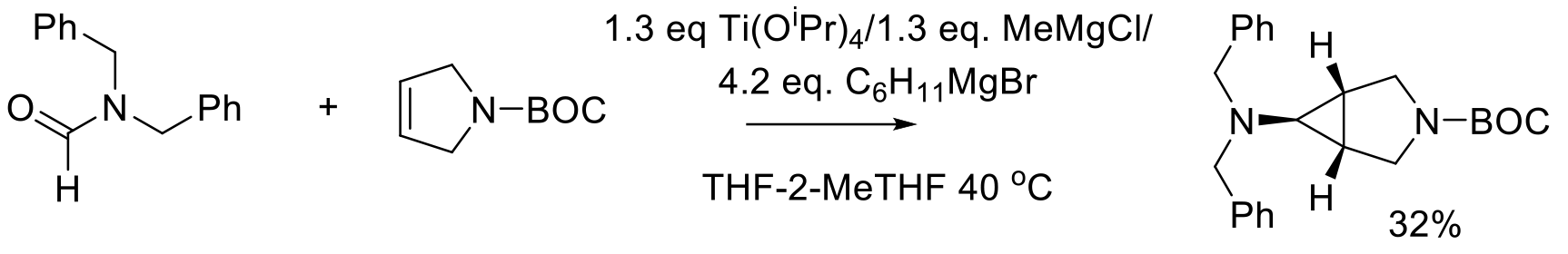
Org. Process Res. Dev. 2018, 22, 728–735.
100 kg scale