Cyclopropanation with Free Carbenes
Mechanism + Description
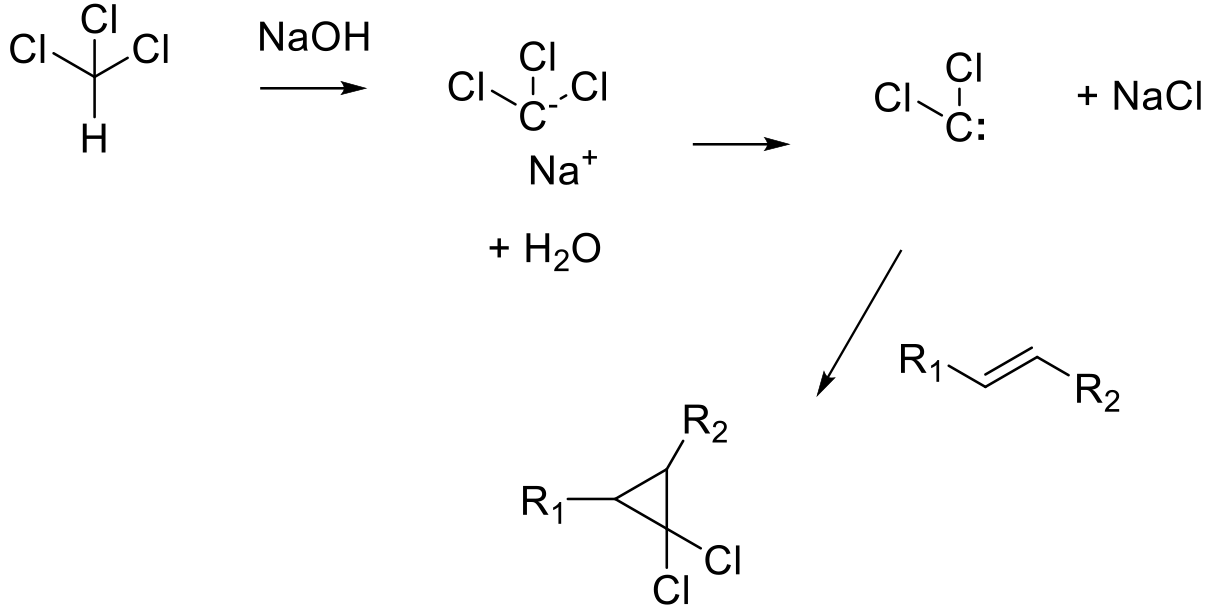
A carbene precursor like diazomethane or chloroform is decomposed to a carbene. In the presence of an alkene substrate, the carbene undergoes a syn addition to the π bond generating cyclopropanes.
General comments
Free carbenes can be used for cyclopropanation reactions, however there is restricted opportunity for this methodology, as few can be produced conveniently in a safe and controlled manner. Most free carbenes are unstable and highly reactive. An exception are dihalocarbenes, such as dichlorocarbene or difluorocarbene, which are relatively stable and will react to form geminal dihalo-cyclopropanes. Most methods used to generate free carbenes are very exothermic making scaling difficult, and reactions are generally unselective with many by-products generated. The use of flow chemistry/photochemistry may help to control reactions with free carbenes and make such processes more attractive for synthesis.
Key references
Relevant scale up examples
None located.