Cyclopropanation Ring Closure via Ionic Mechanism – SN2,Sulfur Ylides
Mechanism + Description
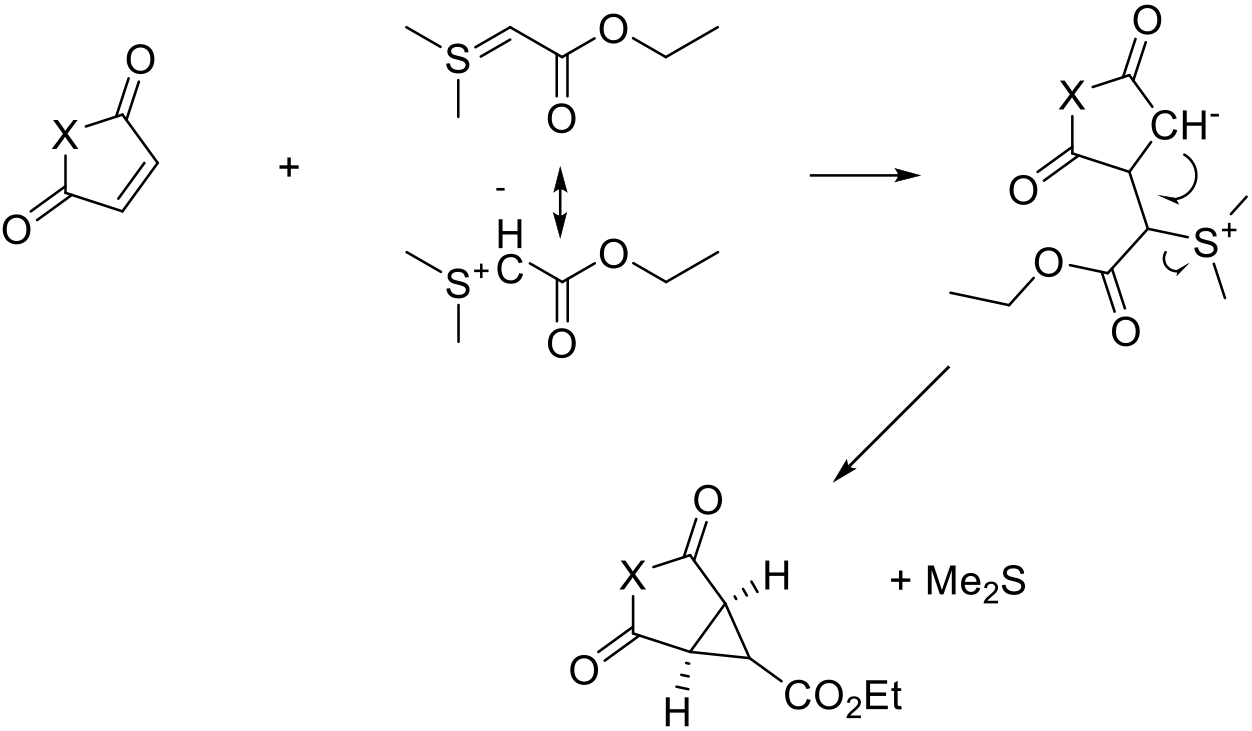
Addition of an anion or anion equivalent (ylide) to an electron poor Michael acceptor followed by a second nucleophilic ring closing reaction generating the cyclopropane ring.
General comments
A number of ionic mechanisms to form cyclopropane rings use an initial nucleophilic addition followed by a second nucleophilic ring closing reaction. This reaction is probably less versatile than the metal catalysed carbene addition since specific structural features of the substrate need to be present, i.e., the Michael acceptor andsite for the addition of the nucleophile. Typical synthons are ylides—usually sulfur-based—in the so-called Corey-Chaykovsky Cyclopropanation (although phosphorus ylides can also be employed). Alternative reagents can be used, e.g., anions with geminal leaving groups such as bromonitromethane.
Key references
Corey, E. J.; Chaykovsky, M. Methylsulfinylcarbanion. J. Am. Chem. Soc. 1962, 84, 866–867.
Johnson, C. R.; Schroeck, C. W. Chemistry of sulfoxides and related compounds. XV. Synthesis of optically active cyclopropanes and oxiranes using an optically active oxosulfonium methylide. J. Am. Chem. Soc. 1968, 90, 6852–6854.
Relevant scale up examples
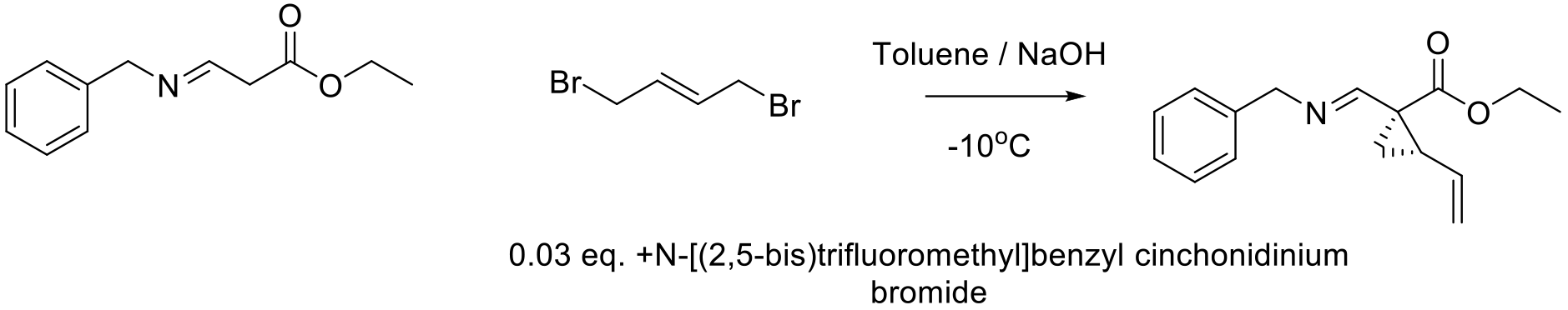
Org. Process Res. Dev. 2010, 14, 692–700.
12g scale
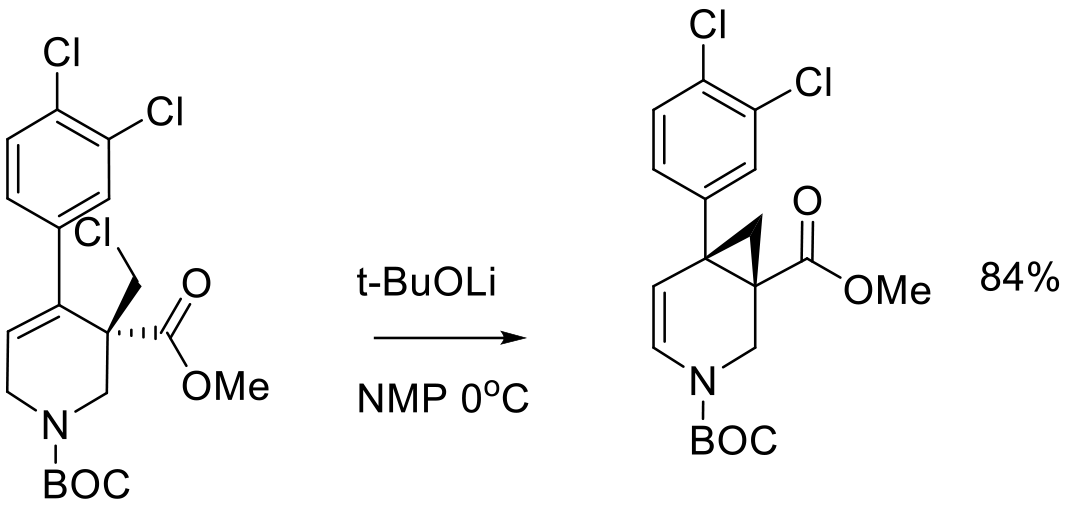
Org. Process Res. Dev. 2010, 14, 912–917.
40 kg scale
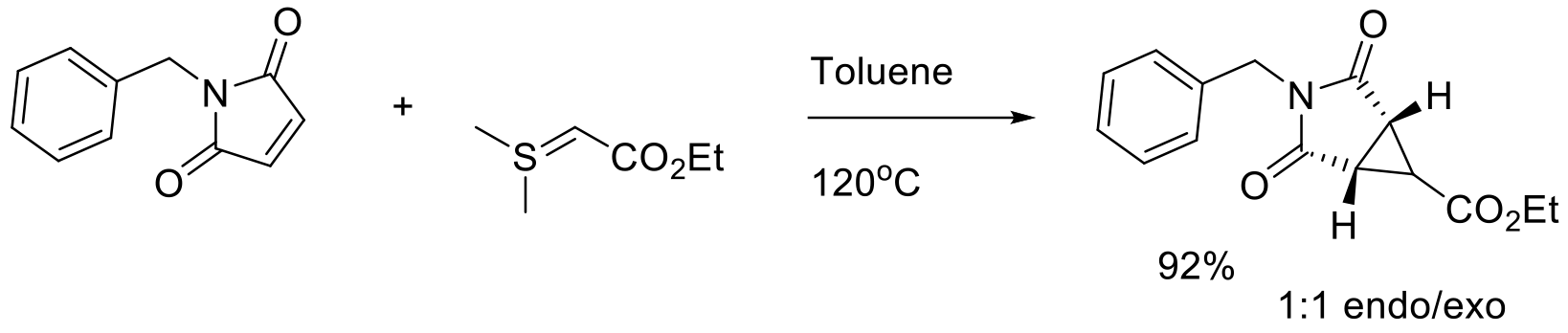
Org. Process Res. Dev. 2014, 18, 1527−1534.
3 kg scale
Green Review
-
Atom efficiency (by-products, molecular weight)
This comes down to the leaving group Cl <Br <I. Sulfates and sulfonic esters produce as high or higher mol. wt. by-products than Cl or Br. Ylides giving Me2S or Me2SO are preferred over P-ylides giving high mol. wt. by-products like Ph3PO. - Safety Concerns
No major safety concerns with the operational aspects of cyclopropanation with SN2/Ylide methodologies - Toxicity and environmental/aquatic impact
No major concerns – by-products like Ph3PO are difficult to biodegrade. - Cost, availability & sustainable feedstocks
Most reagents readily available - Sustainable implications
Na or K bases preferred to Li or Cs bases