TEMPO – Air (O2) Oxidation
Mechanism + Description
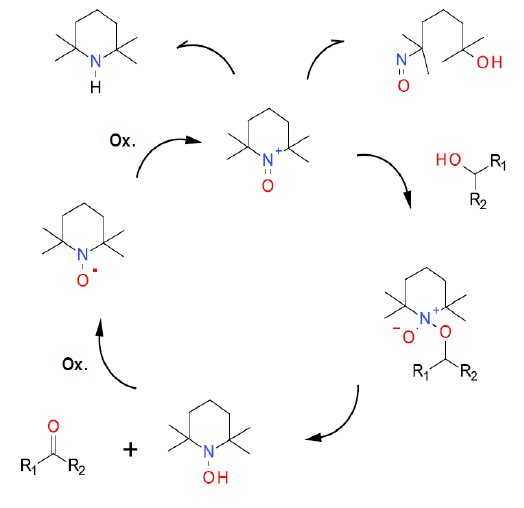 The TEMPO / air mechanism has been studied in depth and reported in the literature (example for benzyl alcohol). The greenest terminal oxidant is air. On its own, this is often kinetically too slow to be practically useful, so it is often employed with co-catalysts like Cu, nitrogen oxides or enzymes (laccase). The oxidation mechanism is identical to other co-oxidants.
The TEMPO / air mechanism has been studied in depth and reported in the literature (example for benzyl alcohol). The greenest terminal oxidant is air. On its own, this is often kinetically too slow to be practically useful, so it is often employed with co-catalysts like Cu, nitrogen oxides or enzymes (laccase). The oxidation mechanism is identical to other co-oxidants.
General comments
A major concern here is the use of flammable solvents in the presence of air. This can be mitigated by the use of flow chemistry/micro reactors and the use of N2/O2 mixtures to keep below the explosion limit. Laccase enzymes work in water but have the drawback of needing relatively high TEMPO loadings.
Key references
Hoover, J. M.; Steves, J. E.; Stahl, S. S. Copper(I)/TEMPO-catalyzed aerobic oxidation of primary alcohols to aldehydes with ambient air. Nature Protocols. 2012, 7 (6), 1161-1166. – practical procedure for CuI/TEMPO-catalyzed air oxidation
Hoover, J. M.; Stahl, S. S. Highly Practical Copper(I)/TEMPO Catalyst System for Chemoselective Aerobic Oxidation of Primary Alcohols. J. Am. Chem. Soc. 2011, 133 (42), 16901-16910. – Review of CuI/TEMPO & air oxidations
He, X.; Shen, Z.; Mo, W.; Sun, N.; Hu, B.; Hu, X. TEMPO-tert-Butyl Nitrate: An Efficient Catalytic System for Aerobic Oxidation of Alcohols. Advanced Synthesis & Catalysis. 2009, 351 (1+2), 89-92. – A metal-free catalytic system for TEMPO and tert-butylnitrite activation of aerobic oxidation
Fabbrini, M.; Galli, C.; Gentili, P.; Macchitella, D. An oxidation of alcohols by oxygen with the enzyme laccase and mediation by TEMPO. Tetrahedron Lett. 2001, 42 (43), 7551–7553. – Oxidation with TEMPO/Laccase & air
Mifsud, M.; Szekrényi, A.; Joglar, J.; Clapés, P. In situ aldehyde generation for aldol addition reactions catalyzed by D-fructose-6-phosphate aldolase. Journal of Molecular Catalysis. 2012, 84, 102-107. – aldehyde oxidation with TEMPO & fructose
Relevant scale-up example
No scale-up examples identified.
Green Review
- Atom efficiency (by-products)
Generally very good – using laccase (and possibly Cu salts) and O2 as the terminal oxidant, the by-product is water. Other co-catalysts may produce peroxide. - Safety concerns
If the procedure requires heat with flammable solvents and air, then caution and controls should be put in place (one option is to limit the % O2 content). If H2O2 is produced as the by-product of O2 reduction, then confirm its compatibility with other components of the reaction mixture. - Toxicity and environmental/aquatic impact
Copper salts are very ecotoxic; enzymes would be an ecologically safer alternative. - Cost, availability & sustainable feedstocks
Air and TEMPO are both readily available. The cost of TEMPO has fallen over time and is now available in bulk. Other analogues are less commercially available and much more expensive, but sometimes exhibit far greater activity. The skeleton of TEMPO comes from acetone and ammonia. - Sustainable implications
Oxygen is the greenest option to partner with TEMPO for catalytic oxidation, but does need a co-catalyst/promotor to get acceptable kinetics.