Me2S/NCS Corey – Kim oxidation
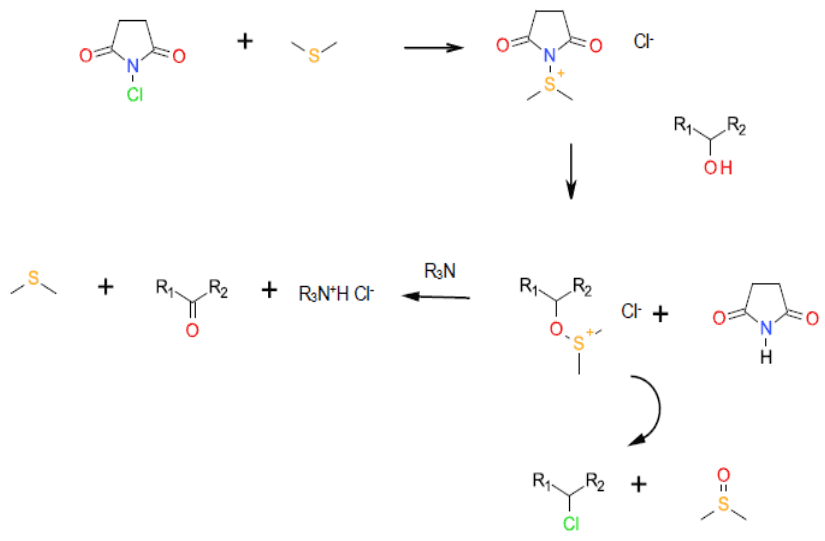
Mechanism + Description
A non-catalytic, non- metal catalysed oxidation mechanistically related to the Swern oxidation, albeit with N-Chlorosuccinimide
Activating the DMS to attack from the alcohol. Chlorinated by-products can result in some instances.
General comments
Often used with complex, multi-functional natural products. A range of ‘greener’ options have been proposed for Swern and Corey-Kim type oxidations. These tend to focus on the use of reagents than remove the odour issues with using/generating Me2S. These alternative S reagents, often solid-supported, are effective at removing issues with volatile sulphides and can facilitate work-up, but add considerably to PMI for a process. It also needs to be borne in mind that making these reagents involves multi-stage synthesis utilising solvents, reagents, energy etc. In mitigation, some can be regenerated and reused.
Key references
Org. Process Res. Dev., 2010, 14 (3), pp 504–510 – 50L scale up of complex macrolide
Green Chemistry (2004), 6(3), 142-146 – A practical improvement of odorless Corey –Kim and Swern oxidations
Tetrahedron (2003), 59(42), 8393-8398 – odorless method for the Corey –Kim utilizing dodecyl methyl sulfide (Dod-S-Me)
Tetrahedron (2002), 58(20), 3865-3870 – fluorous Swern and Corey-Kim reactions: scope and mechanism
Relevant scale up example
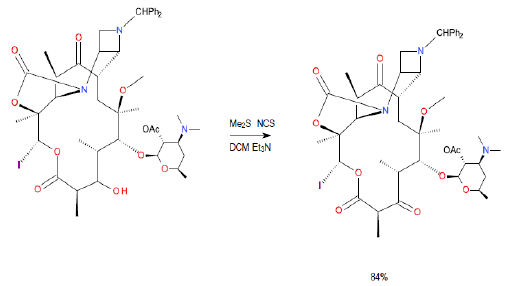
300L scale oxidation
Org. Process Res. Dev., 2012, 16 (5), pp 788–797
Green Review
- Atom efficiency (by-products Mwt)
For removal of H2, the Cory-Kim oxidation produces DMSO (78), Succinimide (99) and HCl (37) as by-products. - Safety Concerns
Me2S reagent and any off gas from the reaction needs to be controlled. The addition of NCS to Me2S and the resultant oxidation step can be highly exothermic. - Toxicity and environmental/aquatic impact
DMS, whilst occurring naturally in the environment, is an irritant, volatile compound with a disagreeable smell. Succinimide is fairly benign. - Cost, availability & sustainable feedstocks
Readily available at reasonable cost. - Sustainable implications
NCS is produced via the chlorination of succinimide with Cl2. Succinimide is produced from succinic acid and ammonia.