NaICl2 A Simple System for the Oxidation of Alcohols
Mechanism + Description
A simple and cheap form of hypervalent iodine, NaICl2, is known to oxidize some alcohols to carbonyl compounds. It is much more atom efficient than IBX or Dess Martin Periodinane, and less hazardous. The iodide is quite electrophilic, and will iodinate electron-rich aromatics and double bonds.
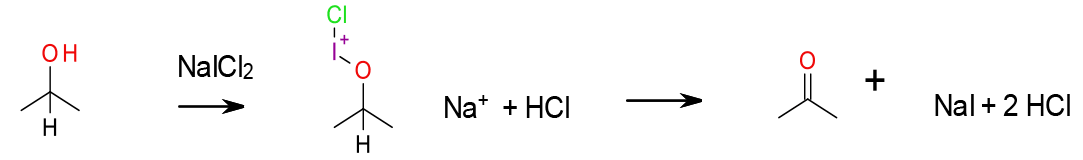
General comments
A simple and mild system for conversion of primary and secondary alcohols to corresponding aldehydes and ketones was developed using aqueous NaICl2 at room temperature in water. This novel application of aqueous NaICl2 gives a high yield and offers a number of advantages in terms of safety and ease of use in comparison to other methods that often employ toxic and hazardous solvents and materials.
Key references
Telvekar, V. N.; Jadhav, N. C. Simple System for Oxidation of Alcohols in Aqueous Solution. Synthetic Communications. 2008, 38 (18), 3107-3111. – oxidation in Aq. systems
Relevant scale-up example
No scale up examples identified
Green Review
- Atom efficiency (by-products)
Moderate – removal of H2 generates inorganic salts(NaI) and HCl – 222 - Safety concerns
No data located – material is an oxidizing agent - Toxicity and environmental/aquatic impact
Toxicity to mammals is unknown. Toxic to aquatic life forms especially in fresh water (iodide). - Cost, availability & sustainable feedstocks
Data unavailable – NaICl2 is a primary product from the recovery of iodine from organoiodide waste. - Sustainable implications
Incineration of waste streams could be problematic (iodine content). Limited utility for waste by-products. Iodine is an element at medium to high risk of depletion. Usually supplied as a solution in water and overall a better choice than DMP or IBX if a hypervalent iodine reagent is needed.