Borylation
The inclusion of an article in this document does not give any indication of safety or operability. Anyone wishing to use any reaction or reagent must consult and follow their internal chemical safety and hazard procedures and local laws regarding handling chemicals
General Overview
Boron reagents are very versatile intermediates in organic synthesis and provide access to advanced carbon-carbon and carbon-heteroatom compounds using mild and catalytic chemistry. Whist the majority of carbon-boron bond forming reactions are performed to insert a reactive handle and the boron is later removed in downstream chemistry, there are some active pharmaceutical agents where the boron is retained as an integral part of the molecule. Several synthetic strategies dominate the chemistry for introduction of boron into a molecule.
- Direct borylation of alkene and alkyne groups (hydroboration).
- Preparation of carbanions and reaction with electrophilic boron reagents.
- Catalytic methods for the borylation of aromatic and heteroaromatic heteroarenes.
In parallel with many other catalytic reaction technologies, there is currently intense interest in developing greener and more sustainable chemistry:
- Combining borylation reactions with a downstream reaction – so called ‘one pot’ or telescoped reactions, e.g. combining borylation and a subsequent Suzuki- Miyaura coupling.
- Use of base metal catalysts to replace precious metal catalysts (Pt group metals).
- Developing catalytic C-H activation methods to directly borylate substrates and avoid halogenated intermediates.
This guide reviews some of the chemistry to make organoboron compounds via the construction of carbon-boron bonds.
General Literature Reviews on Borylation
The vast majority of carbon-boron bond forming reactions are performed to make reagents/substrates for further elaboration. For many transformations, a number of classes of boron reagent could be chosen for that particular downstream coupling reaction. For example, the boron reagents (shown as “phenyl” versions) below have been employed for Suzuki-Miyaura coupling.
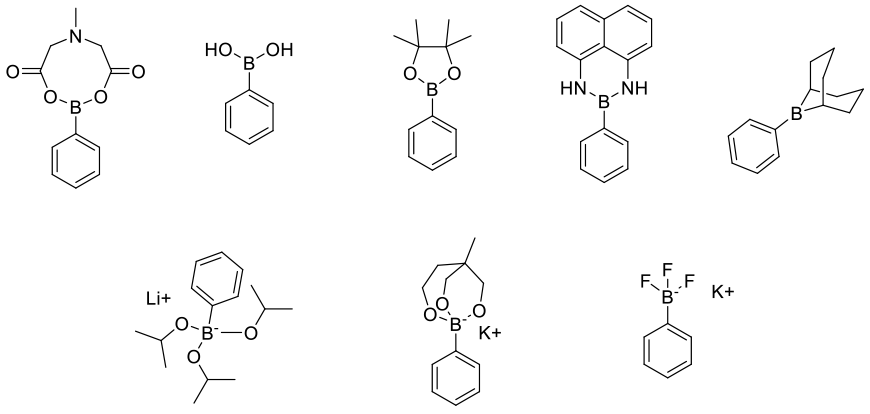
Simplistically, it might be assumed that the most atom-efficient boron reagent would be the best coupling partner, however the selection needs to be driven by looking holistically at the overall process requirements:
- Reactivity demands of the coupling partner
- Extent of side reactions reducing yield and process efficiency, e.g., proto-deborylation, homocoupling, etc.
- Overall process efficiency, e.g., conversion, yields, by-product formation, ease of product isolation etc.
Green Criteria for Borylation
- Large molar excesses of reagents should be avoided if possible.
- Catalytic rather than stoichiometric metal-mediated processes are preferred.
- C-H activation reactions can negate the need for halogenated feedstocks.
- Base metals are preferred to Pt-group metals for catalytic reactions.
- Can borylation reaction be telescoped into the next stage?
- TON/TOF optimised for catalytic reactions.
- Solvents should be chosen to minimize any potential safety and environmental impact.
- Can reactions using hazardous/high reactivity reagents and cryogenic reactions be more efficiently/safely run in flow rather than batch reactors?