Mechanism + Description
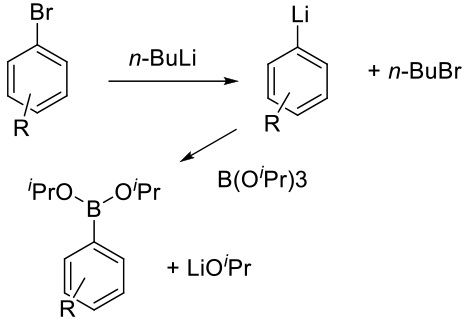
Preparation of a suitable carbanion (Mg, Li, Na) by deprotonation of an aromatic or heteroaromatic substrate, or via metal-halogen exchange and reaction with an appropriate boron-based electrophile.
General comments
Traditional stoichiometric anion chemistry used to make organoboranes. This methodology can be used to make a diverse range of organoboranes and is not limited to aromatic or heteroaromatic compounds. Drawbacks include the use of reactive organometallic bases like alkyllithiums and the use of cryogenic conditions – especially when operated at large scale. There is currently a lot of focus on converting such reactions from batch to flow reactors to enable safer operation, the ability to use potentially unstable organometallic intermediates, and to improve energy metrics by avoiding cryogenic reaction conditions.
Key references
Leonori, D.; Aggarwal, V. K. Lithiation-Borylation Methodology and Its Application in Synthesis. Acc. Chem. Res. 2014, 47, 3174-3183.
Boronic Acids: Preparation and Applications in Organic Synthesis, Medicine and Materials, 1&2; Hall, D. G., Ed.; Wiley-VCH: Weinheim, Germany, 2011; Second Edition.
Leonori, D.; Aggarwal, V. K. Reagent-Controlled Lithiation–Borylation. In Synthesis and Application of Organoboron Compounds; Fernández, E.; Whiting, A., Eds.;Springer: London, 2015; pp 271-295.
Newby, J. A.; Blaylock, D. W.; Witt, P. M.; Turner, R. M.; Heider, P. L.; Harjil, B. H.; Browne, D. L.; Ley, S. V. Reconfiguration of a Continuous Flow Platform for Extended Operation: Application to a Cryogenic Fluorine-Directed ortho-Lithiation Reaction. Org. Process Res. Dev. 2014, 18, 1221−1228.
Usutani, H.; Cork, D. G. Effective Utilization of Flow Chemistry: Use of Unstable Intermediates, Inhibition of Side Reactions, and Scale-Up for Boronic Acid Synthesis. Org. Process Res. Dev. 2018, 22, 741−746.
Usutani, H.; Nihei, T.; Papgeorgiou, C. D.; Cork, D. G. Development and Scale-up of a Flow Chemistry Lithiation−Borylation Route to a Key Boronic Acid Starting Material. Org. Process Res. Dev. 2017, 21, 669−673.
Desai, A. A. Overcoming the Limitations of Lithiation Chemistry for Organoboron Compounds with Continuous Processing. Angew. Chem. Int. Ed. 2012, 51, 9223-9225.
Broom, T.; Hughes, M.; Szczepankiewicz, B. G.; Ace, K.; Hagger, B.; Lacking, G.; Chima, R. Marchbank, G.; Alford, G.; Evans, P.; et al. The Synthesis of Bromomethyltrifluoroborates through Continuous Flow Chemistry. Org. Process Res. Dev. 2014, 18, 1354–1359.
Relevant scale up example
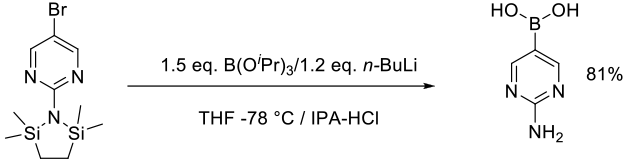
Patel, N. D.; Zhang, Y.; Gao, J.; Sidhu, K.; Lorenz, J. C.; Fandrick, K. R.; Mulder, J. A.; Herbage, M. A.; Li, Z.; Ma, S.; et al. Org. Process Res. Dev. 2016, 20, 95−99.

Ashworth, I. W.; Campbell, A. D.; Cherryman, J. H.; Clark, J.; Crampton, A.; Eden-Rump, E. G. B.; Evans, M. Jones, M. F.; McKeever-Abbas, S.; Meadows, R. E.; et al. Org. Process Res. Dev. 2018, 22, 1801-1808.
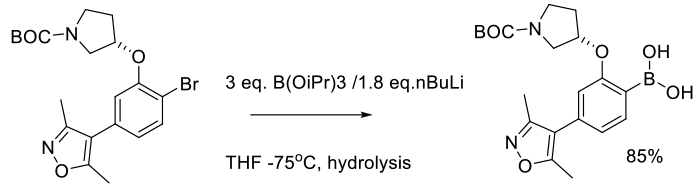
Murugan, A.; Bachu, S.; Manjunatha, S. G.; Ramakrishnan, R.; Kadamar, V. K.; Reddy, C.; Torlikonda, V. R.; George, S.; Ramasubramanian, S.; Nambiar, S. Org. Process Res. Dev. 2014, 18, 646−651.
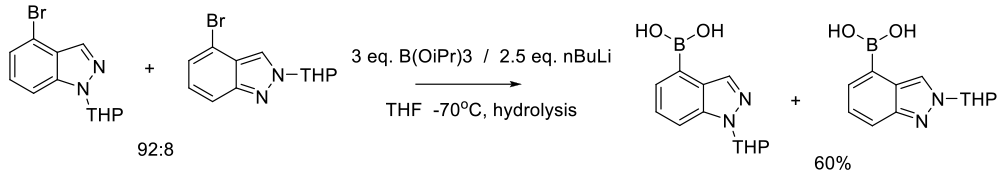
Tian, Q.; Cheng, Z.; Yajima, H. M.; Savage, S. J.; Green, K. L.; Humphries, T.; Reynolds, M. E.; Babu, S.; Gosselin, F.; Askin, D.; et. al. Org. Process Res. Dev. 2013, 17, 97-107.
Green Review
-
Atom efficiency (by-products Mwt)
Reactions will generate an alkane or alkyl halide as a by-product of the metalating agent and a metal salt by-product.
- Safety Concerns
Organometallic reactions can be very exothermic and use highly flammable reagents like n-BuLi. Metallated intermediates can be unstable at room temperature. Reactions can degas flammable gas (e.g. n-butane) on quenching.
- Toxicity and environmental/aquatic impact
The main concern is around the loss of boric acid/organoboron reagents into waste streams.
Fluoride toxicity to aquatic organisms: a review.
- Cost, availability & sustainable feedstocks
Most reagents are readily available at scale.
- Sustainable implications
Li is at medium risk of depletion. Large scale cryogenic reactions are not energy efficient.




