Mechanism + Description
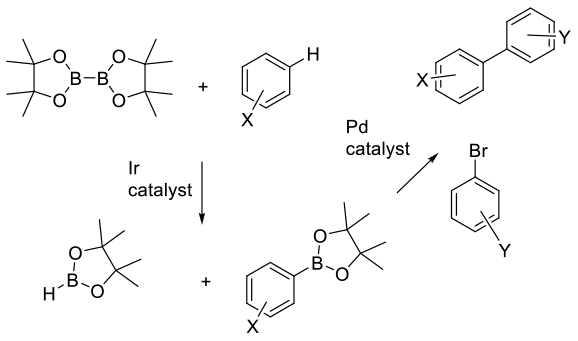
Essentially a ‘one pot’ C-borylation followed by a second catalyzed or uncatalyzed reaction giving the desired product.
General comments
It is becoming more common to see borylation reactions being run as ‘telescoped’ or ‘one pot’ processes. Since it is usual for the borylated product to be further transformed, a ‘one pot’ process generally avoids isolations, reduces the use of organic solvents and gives processes with better process mass intensity (PMI) metrics. It is unusual to find true ‘one pot’ processes where all reagents/catalysts are added at the start. More commonly is to essentially run two consecutive reactions; first forming the boryl compound, then adding fresh reagents and (often discrete) catalysts.
Key references
See also Suzuki-Miyaura reagent guide
Molander, G. A.; Trice, S. L. J.; Kennedy, S. M. Scope of the Two-Step, One-Pot Palladium-CatalyzedBorylation/Suzuki Cross-Coupling Reaction Utilizing Bis-Boronic Acid. J. Org. Chem. 2012, 77, 8678–8688.
Cinelli, M. A.; Li, H.; Chreifi, G.; Poulos, T. L.; Silverman, R. B. Nitrile in the Hole: Discovery of a Small Auxiliary Pocket in Neuronal Nitric Oxide Synthase Leading to the Development of Potent and Selective 2-Aminoquinoline Inhibitors. J. Med. Chem. 2017, 60, 3958-3978.
Xu, L.; Li, P. Differentiated Di- and Polyboron Compounds: Synthesis and Application in Successive Suzuki–Miyaura Coupling. Synlett 2014, 5, 1799-1802.
Xu, S. D.; Sun, F. Z.; Deng, W. H.; Hao, H.; Duan, X. H. One-Step Highly Selective Borylation/Suzuki Cross-Coupling of Two Distinct Aryl Bromides in Pure Water. New J. Chem. 2018, 42, 16464-16468.
Ji, H.; Wu, L.-Y.; Cai, J.-H.; Li, G.-R.; Gan, N.-N.; Wang, Z.-H. Room-Temperature Borylation and One-Pot Two-Step Borylation/Suzuki-Miyaura Cross-Coupling Reaction of Aryl. RSC Adv. 2018, 8, 13643-13648.
Pandarus, V.; Gingras, G.; Béland, F.; Ciriminna, R.; Pagliaro, M. Clean and Fast Cross-Coupling of Aryl Halides in One-Pot. Beilstein J. Org. Chem. 2014, 10, 897-901.
Dzhevakov, P. B.; Topchiy, M. A.; Zharkova, D. A.; Morozov, O. S.; Asachenko, A. F.; Nechaev, M. S. MiyauraBorylation and One-Pot Two-Step Homocoupling of Aryl Chlorides and Bromides Under Solvent-Free Conditions. Adv. Synthesis & Catal. 2016, 358, 977-983.
Merkul, E.; Schäfer, E.; Müller, T. Rapid synthesis of bis(hetero)aryls by one-pot Masuda borylation–Suzuki coupling sequence and its application to concise total syntheses of meridianins A and G. J. Org. Biomol. Chem. 2011, 9, 3139-3141.
Klečka, M.; Slavětínská, L. P.; Hocek, M. Modification of Pyrrolo[2,3-d]pyrimidines by C–H Borylation Followed by Cross-Coupling or Other Transformations: Synthesis of 6,8-Disubstituted 7-Deazapurine Bases. EurJOC 2015, 36, 7943–7961.
Pubill-Ulldemolins, C.; Bonet, A.; Bo, C.; Gulyás, H.; Fernández, E. A New Context for Palladium Mediated B-Addition Reaction: An Open Door to Consecutive Functionalization. Org. Biomol. Chem. 2010, 8, 2667-2682.
Egan, B. A.; Burton, P. M. Synthesis of 3-Aryl-1H-Indazoles via Iridium-Catalysed C–H Borylation and Suzuki–Miyaura Coupling. RSC Adv. 2014, 4, 27726-27729.
Homer, J. A.; Sperry, J. A Short Synthesis of the Endogenous Plant Metabolite 7-Hydroxyoxindole-3-Acetic Acid (7-OH-OxIAA) Using Simultaneous C–H Borylations. Tetrahedron Lett. 2014, 55, 5798-5800.
Whitaker, L. Harb, H. Y.; Pulis, A. P. One-Pot Borylation/Suzuki–Miyaura sp2–sp3 Cross-Coupling. Chem. Commun. 2017, 53, 9364-9367.
Tzschucke, C. C.; Murphy, J. M.; Hartwig, J. F. Arenes to Anilines and Aryl Ethers by Sequential Iridium-CatalyzedBorylation and Copper-Catalyzed Coupling. Org. Lett. 2007,9, 761–764.
Kallepalli, V. A.; Sánchez, L.; Li, H.; Gesmundo, N. J.; Turton, C. L.; Maleczka, Jr., R. E.; Smith, III, M. R. Divergent Synthesis of 2,3,5-Substituted Thiophenes by C-H Activation/Borylation/Suzuki Coupling, Heterocycles 2010, 80, 1429-1448.
Holmes, D.; Chotana, G. A.; Maleczka, R. E.; Smither, M. R. One-Pot Borylation/Amination Reactions: Syntheses of ArylamineBoronate Esters from Halogenated Arenes. Org. Lett. 2006, 8, 1407-1410.
Relevant Scale-up Examples – ‘One-pot’ Borylation and Functionalisation
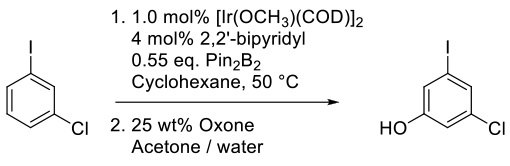
Campeau, L.-C.; Chen, Q.; Gauvreau, D.; Girardin, M.; Belyk, K.; Maligres, P.; Zhou, G.; Gu, C.; Zhang, W.; Tan, L. O’Shea, P. D. A Robust Kilo-Scale Synthesis of Doravirine. Org. Process Res. Dev. 2016, 20, 1476-1481.
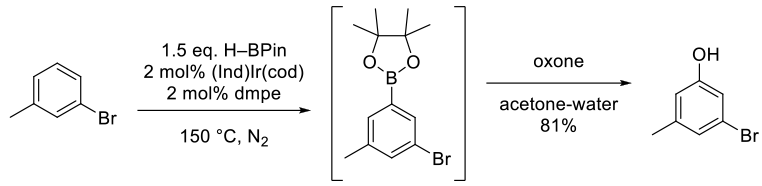
Norberg, A. M.; Smith III, M. R.; Maleczka, Jr., R. E. Practical One-Pot C-H Activation/Borylation/Oxidation: Preparation of 3-Bromo-5-Methylphenol on a Multigram Scale. Synthesis 2011, 6, 857-859.


