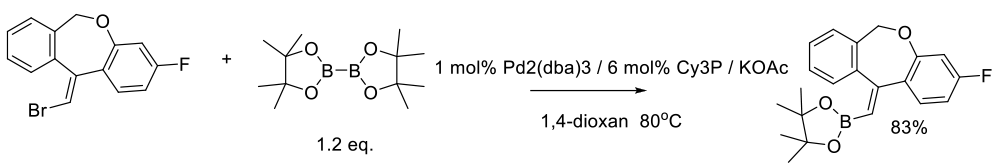Mechanism + Description
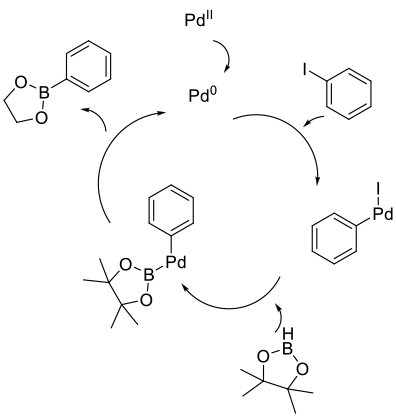
Mirrors classical Buchwald-Hartwig type coupling to give amines/ethers/thioethers. Oxidative addition of a Pt-group metal catalyst
into an arene/heteroaromatic/alkene–halide bond followed by the usual reductive elimination to give the desired product.
General comments
Miyaura borylation – a catalytic route to organoboranes avoiding the use of stoichiometric reagents like alkyllithiums.
Typical substrates are halides, e.g., I, Br, Cl and sulfonates. The catalyst is normally a Pd source with an appropriate
ligand and boron coupling partner. The choice of ligand is a crucial factor in getting an efficient catalytic reaction.
The reaction is run in the presence of a base. Bases are typically inorganics like NaOH, Na/K/Cs2CO3, Na/KHCO3
and KXHYPO4. Occasionally organic amine bases are employed.
Key references
Haddenham, D.; Bailey, C. L.; Vu, C.; Nepomuceno, G.; Eagon, S.; Pasumansky, L.; Singararm, B. Lithium aminoborohydrides 17. Palladium catalyzedborylation of aryl iodides, bromides, and triflates with diisopropylaminoborane prepared from lithium diisopropylaminoborohydride. Tetrahedron 2011, 67, 576-583.
Takagi, J.; Takahashi, K.; Ishiyama, T.; Miyaura, N. Palladium-Catalyzed Cross-Coupling Reaction of Bis(pinacolato)diboron with 1-Alkenyl Halides or Triflates: Convenient Synthesis of Unsymmetrical 1,3-Dienes via the Borylation-Coupling Sequence. J. Am. Chem. Soc. 2002, 124, 8001-8006.
Molander, G. A.; Trice, S. L. J.; Kennedy, S. M.; Dreher, S. D.; Tudge, M. T. Scope of the Palladium-Catalyzed Aryl Borylation Utilizing Bis-Boronic Acid. J. Am. Chem. Soc. 2012, 134, 11667–11673.
Vogels, C. M.; Westcott, S. A. Sterically Demanding Aryl Chlorides: No Longer a Problem for Borylations. ChemCatChem 2012, 4, 47-49.
Zernickel, A.; Du, W.; Ghorpade, S. A.; Sawant, D. N.; Makki, A. A.; Sekar, N.; Eppinger, J. Bedford-Type Palladacycle-CatalyzedMiyauraBorylation of Aryl Halides with Tetrahydroxydiboron in Water. J. Org. Chem. 2018, 83, 1842-1851.
Molander, G. A.; Trice, S. L. J.; Kennedy, S. M. Palladium-CatalyzedBorylation of Aryl and Heteroaryl Halides Utilizing Tetrakis(dimethylamino)diboron. Org. Lett. 2012, 14, 4814-4817.
Guerrand, H. D. S.; Vaultier, M.; Pinet, S.; Pucheault, M. Amine–Borane Complexes: Air- and Moisture-Stable Partners for Palladium-CatalyzedBorylation of Aryl Bromides and Chlorides. Adv. Synth. Catal. 2015, 357, 1167–1174.
Murata, M.; Oyama, T.; Watanabe, S.; Masuda, Y. Palladium-CatalyzedBorylation of Aryl Halides or Triflates with Dialkoxyborane: A Novel and Facile Synthetic Route to Arylboronates. J. Org. Chem. 2000, 65, 164-168.
Billingsley, K. L.; Buchwald, S. L. An Improved System for the Palladium-CatalyzedBorylation of Aryl Halides with Pinacol Borane. J. Org. Chem. 2008, 73, 5589-5591.
Marata, M. Oyama, T.; Watanabe, S.; Masuda, Y. Palladium-CatalyzedBorylation of Aryl Halides or Triflates with Dialkoxyborane: A Novel and Facile Synthetic Route to Arylboronates. J. Org. Chem .2000, 65, 164-168.
Relevant Scale-up Examples of Miyaura Borylation

Smith, M. J.; Lawler, M. J.; Kopp, N.; Mcleod, D. D.; Davulcu, A. H.; Lin, D.; Kitipally, K.; Sfouggatakis, C. Org. Process Res. Dev. 2017, 21, 1859–1863

Edney, D.; Hulcoop, D. G.; Leahy, J. H.; Vernon, L. E.; Wipperman, M. D.; Bream, R. N.; Webb, M. R. Org. Process Res. Dev. 2018, 22, 368−376.
Patel, N. D.; Zhang, Y.; Gao, J.; Sidhu, K.; Lorenz, J. C.; Fandrick, K. R.; Mulder, J. A.; Herbage, M. A.; Li, Z.; Ma, S.; et. al. Org. Process Res. Dev. 2016, 20, 95−99

Gurung, S. R.; Mitchell, C.; Huang, J.; Jonas, M.; Strawser, J. D.; Daia, E.; Hardy, A.; O’Brien, E.; Hicks, F.; Papageorgiou, C. D. Org. Process Res. Dev. 2017, 21, 65–74.
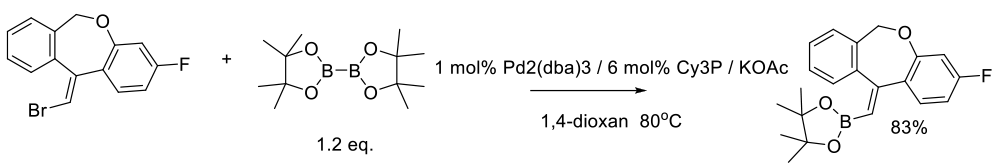
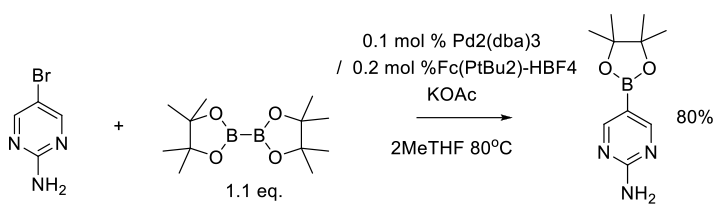
Hansen, M. M.; Kallman, N. J.; Koenig, T. M.; Linder, R. J.; Richey, R. N.; Rizzo, J. R.; Ward, J. A.; Yu, H.; Zhang, T. Y.; Mitchell, D. Org. Process Res. Dev. 2017, 21, 208−217.
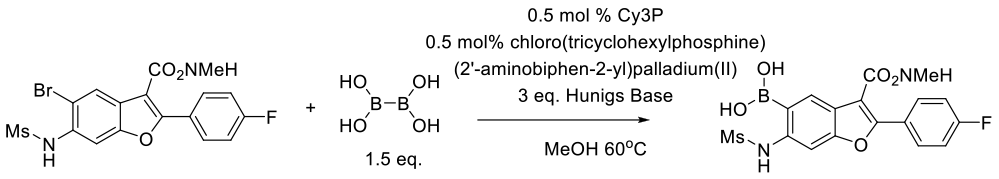
Williams, M. J.; Chen, Q.; Codan, L.; Dermenjian, R. K.; Dreher, S.; Givson, A. W.; He, X.; Jin, Y.; Keen, S. P.; Lee, A. Y. Org. Process Res. Dev. 2016, 20, 1227−1238.
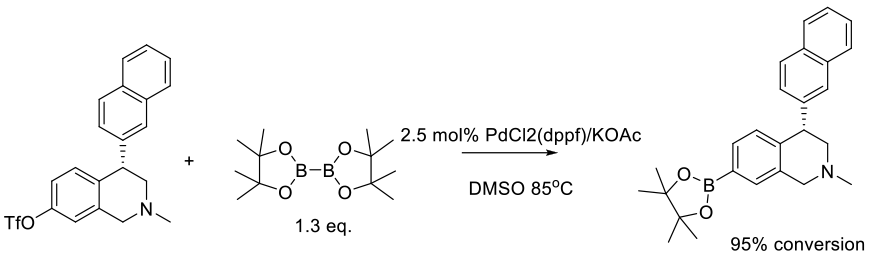
Lobben, P. C.; Amin, R.; Chen, B.-C.; Cui, W.; Hu, M.; Isherwood, M.; Liu, S.; Nacro, K.; Miles, B.; Mobele, B.; et. al. Org. Process Res. Dev. 2016, 20, 44−50.