Mechanism + Description
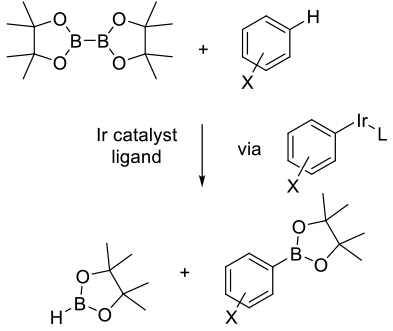
Similar to Pd-catalyzed mechanism except prior activation of the target bond by conversion to a halide or pseudohalide is not required, and direct insertion of the metal-ligand catalyst occurs into a C-H bond.
General comments
In order to reduce the reliance on halogenated substrates typically employed in metal-catalysed carbon heteroatom
bond formation, direct introduction of the boron functionality via C-H activation chemistry is currently an area of
intense interest. Typically using precious metal catalysts like Ir, Ru, and Rh, there is also interest in also employing
base metal catalysts. Key to a productive and high-yielding C-H activation is control over the regioselectivity and
avoiding the formation of isomeric products that need to be separated. Selectivity is driven by a number of parameters:
- Steric and electronics on the substrate
- Presence of directing groups on the substrate
- Reactivity of the active catalyst species imparted by certain ligands
Key references
Okada, S.; Namikoshi, T.; Watanabe, S.; Murata, M. Ruthenium-Catalyzed Ortho-Selective Aromatic C-H Borylation of 2-Arylpyridines with Pinacolborane. ChemCatChem 2015, 7, 1531–1534.
Liskey, C. W.; Hartwig, J. F. Iridium-Catalyzed C–H Borylation of Cyclopropanes. J. Am. Chem. Soc. 2013, 135, 3375–3378.
Sadler, S. A.; Hones, A. C.; Roberts, B.; Blakemore, D.; Marder, T. B.; Steel, P. G. Multidirectional Synthesis of Substituted Indazoles via Iridium-Catalyzed C–H Borylation . J. Org. Chem. 2015, 80, 5308-5314.
Murphy, J. M.; Tzschucke, C. C.; Hartwig, J. F. One-Pot Synthesis of Arylboronic Acids and Aryl Trifluoroborates by Ir-CatalyzedBorylation of Arenes. Org. Lett. 2007, 9, 757–760.
Preshlock, S. M.; Ghaffari, B.; Maligres, P. E.; Krska, S. W.; Maleczka, Jr., R. E.; Smith, III, M. R. High-Throughput Optimization of Ir-Catalyzed C–H Borylation: A Tutorial for Practical Applications. J. Am. Chem. Soc. 2013, 135, 7572-7582.
Larsen, M. A.; Hartwig, J. F. Iridium-Catalyzed C–H Borylation of Heteroarenes: Scope, Regioselectivity, Application. J. Am. Chem. Soc. 2014, 136, 4287-4299.
Hartwig, J. F. Catalyst-Controlled Site-Selective Bond Activation. Acc. Chem. Res. 2017, 50, 549-555.
Cernak, T.; Dykstra, K. D.; Tyagarajan, S.; Vachal, P.; Krska, S. W. The Medicinal Chemist’s Toolbox for Late Stage Functionalization of Drug-Like Molecules. Chem. Soc. Rev. 2016, 45, 546-576.
Su, B.; Zhou, T.-G.; Xu, P.L.; Shi, Z.-J.; Hartwig, J. F. Enantioselective Borylation of Aromatic C−H Bonds with Chiral Dinitrogen Ligands. Angew. Chem., Int. Ed. 2017, 56, 7205-7208.
Wu, F.; Feng, Y.; Jones, C. W. Recyclable Silica-Supported Iridium Bipyridine Catalyst for Aromatic C–H Borylation. ACS Catal. 2014, 4, 1365–1375.
Preshlock, S. M.; Plattner, D. L.; Maligres, P. E.; Krska, S. W.; Maleczka, Jr., R. E.; Smith, III, M. R. A Traceless Directing Group for C-H Borylation. Angew. Chem., Int. Ed. 2013, 52, 12915–12919.
Press, L. P.; Kosanovich, A. J.; McCulloch, B. J.; Ozerov, O. V. High-Turnover Aromatic C–H BorylationCatalyzed by POCOP-Type Pincer Complexes of Iridium. J. Am. Chem. Soc. 2016, 138, 9487-9497.
Kawamorita, S.; Ohmiya, H.; Hara, K.; Fukuoka, A.; Sawamura, M. Directed Ortho Borylation of Functionalized ArenesCatalyzed by a Silica-Supported Compact Phosphine−Iridium System. J. Am. Chem. Soc. 2009, 131, 5058–5059.
Larsen, M. A.; Cho, S. H.; Hartwig, J. Iridium-Catalyzed, Hydrosilyl-Directed Borylation of Unactivated Alkyl C-H Bonds. J. Am. Chem. Soc. 2016,138, 762–765.
Kallepalli, V. A.; Shi, F.; Paul, S.; Onyeozili, E. N.; Maleczka, R. E.; Smith, III, M. R. Boc Groups as Protectors and Directors for Ir-Catalyzed C-H Borylation of Heterocycles. J. Org. Chem. 2009,74, 9199-9201.
Bheeter, C. B.; Chowdhury, A. D.; Adam, R.; Jackstell, R.; Beller, M. Efficient Rh-catalyzed C–H borylation of arene derivatives under photochemical conditions. Org. Biomol. Chem. 2015, 13, 10336-10340.
Bisht, R.; Chattopadhyay, B. ortho- and meta-Selective C–H Activation and Borylation of Aromatic Aldehydes via in situ Generated Imines. Synlett 2016, 27, 2043-2050.
Sadler, S. A.; Tajuddin, H.; Mkhalid, I. A.; Batsanov, A. S.; Albesa-Jove, D.; Cheung, M. S.; Maxwell, A. C.; Shukla, L.; Roberts, B.; Blakemore, D. C. Iridium-Catalyzed C–H Borylation of Pyridines. Org. Biomol. Chem. 2014, 12, 7318-7327.
Hurst, T. E.; Macklin, T. K.; Becker, M.; Hartmann, E.; Kügel, W.; Parisienne-La Salle, J.-C.; Batsanov, A. S.; Marder, T. B.; Snieckus, V. Iridium-Catalyzed C-H Activation versus Directed ortho Metalation: Complementary Borylation of Aromatics and Heteroaromatics. Chem. Eur. J. 2010,16, 8155–8161.
Tajuddin, H.; Harrisson, P. Collings, J. C.; Sim, N.; Batsanov, A. S.; Cheung, M. S.; Kawamorita, S.; Maxwell, A. C.; Shukla, L.; et. al. Iridium-catalyzed C–H borylation of quinolines and unsymmetrical 1,2-disubstituted benzenes: insights into steric and electronic effects on selectivity. Chem. Sci. 2012, 3, 3505-3515.
Kawamorita, S.; Ohmiya, H.; Sawamura, M. Ester-Directed RegioselectiveBorylation of HeteroarenesCatalyzed by a Silica-Supported Iridium Complex. J. Org. Chem. 2010, 75, 3855–3858.
Tagata, T.; Nishida, M.; Nishida, A. Development of Recyclable Iridium Catalyst for C-H Borylation. Tetrahedron Lett. 2009, 50, 6176-6179.
Larsen, M. A.; Hartwig, J. F. Iridium-Catalyzed C–H Borylation of Heteroarenes: Scope, Regioselectivity, Application to Late-Stage Functionalization, and Mechanism. J. Am. Chem. Soc. 2014, 136, 4287–4299.
Seechurn, C. C. C. J.; Sivakumar, V.; Satoskar, D.; Colacot, T. J. Iridium-Catalyzed C–H Borylation of Heterocycles Using an Overlooked 1,10-Phenanthroline Ligand: Reinventing the Catalytic Activity by Understanding the Solvent-Assisted Neutral to Cationic Switch. Organometallics 2014,33, 3514–3522.
Sadler, S. A.; Tajuddin, H.; Mkhalid, I. A. I.; Batsanov, A. S.; Albesa-Jove, D.; Cheung, M. S.; Maxwell, A. C.; Shukla, L.; Roberts, B. Iridium-Catalyzed C–H Borylation of Pyridines. Org. Biomol. Chem. 2014, 12, 7318-7327.
Sadler, S. A.; Hones, A. C.; Roberts, B.; Blakemore, D.; Marder, T. B.; Steel, P. G. Multidirectional Synthesis of Substituted Indazoles via Iridium-Catalyzed C–H Borylation, J. Org. Chem. 2015, 80, 5308–5314.
Saito, Y.; Segawa, Y.; Itami, K. para-C–H Borylation of Benzene Derivatives by a Bulky Iridium Catalyst. J. Am. Chem. Soc. 2015, 137, 5193–5198.
Feng, Y.; Holte, D.; Zoller, J.; Umemiya, S.; Simke, L. R.; Baran, P. S. Total Synthesis of Verruculogen and Fumitremorgin A Enabled by Ligand-Controlled C–H Borylation. J. Am. Chem. Soc. 2015, 137, 10160–10163.
Wang, G.; Xu, L.; Li, P. Double N,B-Type Bidentate Boryl Ligands Enabling a Highly Active Iridium Catalyst for C–H Borylation J. Am. Chem. Soc. 2015, 137, 8058–8061.
Cho, S. H.; Hartwig, J. F. Iridium-CatalyzedBorylation of Secondary Benzylic C–H Bonds Directed by a Hydrosilane. J. Am. Chem. Soc. 2013, 135, 8157–8160.
Relevant scale up example – C-H borylation
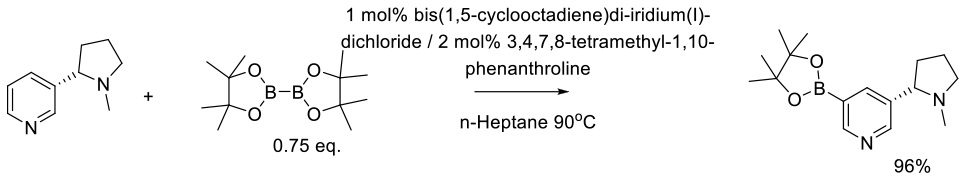
Sieser, J. E.; Maloney, M. T.; Chisowa, E.; Brenek, S. J.; Monfette, S.; Salisbury, J. J.; Do, N. M.; Singer, R. A. Org. Process Res. Dev. 2018, 22, 527–534.

