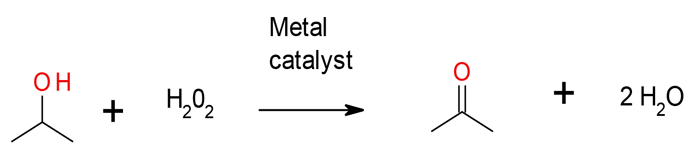
Activated H2O2 Hydrogen Peroxide
Mechanism + Description
 The mechanism may vary with the metal and homo / hetero nature
of the catalyst.With homogeneous catalysts, several reports have described
That O2 generated via decomposition of H2O2 is not the oxidant. For homogeneous reactions, the active catalyst is probably a peroxy-metal species.
The mechanism may vary with the metal and homo / hetero nature
of the catalyst.With homogeneous catalysts, several reports have described
That O2 generated via decomposition of H2O2 is not the oxidant. For homogeneous reactions, the active catalyst is probably a peroxy-metal species.
General comments
The method of activating hydrogen peroxide/hydroperoxides usually on metal catalysts is a variant on the use of air/O2
The reaction can be carried out under mild conditions, but background decomposition of peroxide or hydroperoxide can lead to higher than stoichiometric demands for the oxidant. The catalyst –peroxide oxidation can show selectivity for the oxidation of secondary alcohols in the presence of primary alcohols. Other functional groups may be oxidized.
Key references
Green Chemistry (2009), 11(6), 756-759 – Green and efficient oxidation using aq.H2O2 and gold catalysis
Organic Letters (2010),12 (20) 4540-4543 – polymeric phosphotungstate catalysis
Green Chemistry Letters and Reviews (2012), 5(2), 195-202 – Titanium (IV) oxide in polyethylene glycol oxidation
Synthetic Communications (2012), 42(3), 299-308 – Oxid’n using TBHP over LaMO3 perovskites (M = Fe, Ni, Co, Mn, Cr)
Relevant scale up example
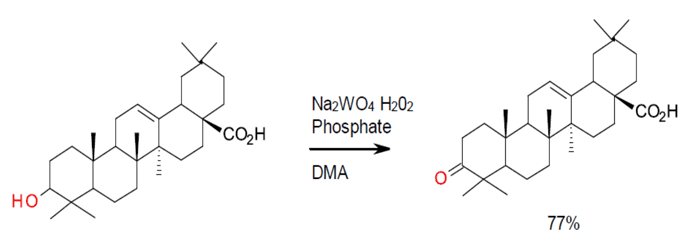
Org. Process Res. Dev., 2010, 14 (1), pp 289–294
Green Review
-
Atom efficiency (by-products Mwt)
The exact atom efficiency cannot be determined given the range of metal-air conditions, however hydrogen peroxide is the next best oxidant to molecular oxygen. Atom efficiency will be reduced with higher order peroxides, and if the catalyst shows catalysis –type side activity and decomposed peroxide to oxygen, mass efficiency will also suffer. - Safety Concerns
Potential for highly exothermic and delayed exotherm reactions so should be scaled with caution. The use of hydrogen peroxide should be limited to 30% wt or less solutions. Organohydroperoxides can be heat and shock sensitive. If using flammable solvents, care should be given to the fact that a flammable and explosive mixture could be generated if O2 is evolved as a side reaction. - Toxicity and environmental/aquatic impact
Generally good – by-products from H2O2 and excess H2O2 are innocuous to the environment. Any major concerns would come from the solvent used and the potential for any ecotoxic metal catalyst to be discharged into aqueous waste streams. - Cost, availability & sustainable feedstocks
Many of the metals outlined are simple complexes with good commercial availability on scale, however some of them are more novel (nano-clusters, complexes etc) that are in their infancy. - Sustainable implications
With good catalytic efficiency, and recovery of the catalyst if precious metal, only water is generated as a by-product if H2O2 is used – this reagent is preferred to hydroperoxide oxidants. The use of base metals is preferred to precious metals if at all possible. Many current publications focus on Au as a catalyst. This metal has a very high LCA to produce. Although very cheap, H2O2 has a reasonable LCI since its manufacture is a complex process (can not be produced directly from O2 and H2).