Dess Martin Periodate
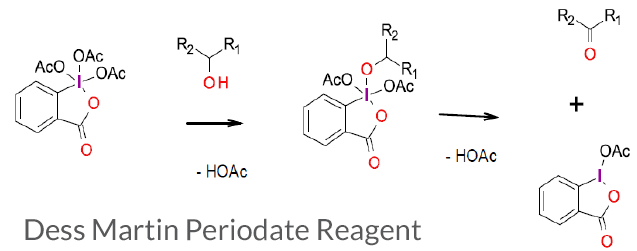
Mechanism + Description
The Dess Martin reagent is very similar to IBX in reactivity. See comments for IBX.
General comments
Dess–Martin periodinane (DMP) is a hypervalent iodine reagent used to oxidize primary alcohols to aldehydes and secondary alcohols to ketones. This reagent is attractive because of its operation under mild conditions (room temperature, neutral pH), short reaction times, higher yields, simple work-ups, high chemoselectivity, tolerance of sensitive functional groups, and a long shelf life. The presence of acetate groups makes DMP more soluble in a wider range of solvents compared to the IBX reagent.
Key references
Boeckman, R. K. Jr.; Shao, P.; Mullins, J. J. The Dess-Martin Periodinane: 1, 1, 1-Triacetoxy-1,1-Dihydro-1 Benziodoxol-3(1H)-One [1,2-Benziodoxol-3(1H)-one, 1,1,1-tris(acetyloxy)-1,1-dihydro-]. Org. Synth. 2000, 77, 141. – Review of DMP
Mickel, S. J.; Niederer, D.; Daeffler, R.; Osmani, A.; Kuesters, E.; Schimd, E.; Schaer, K.; Gamboni, R.; Chen, W.; Loeser, E.; et al. Large-Scale Synthesis of the Anti-Cancer Marine Natural Product (+)- Discodermolide. Part 5: Linkage of Fragments C1-6 and C7-24 and Finale. Org. Process Res. Dev. 2004, 8 (1), 122-130. – Gram scale example on Discodermolide
Loiseleur, O.; Schneider, H.; Huang, G.; Machaalani, R.; Sellès, P.; Crowley, P.; Hanessian, S. A Scalable Synthesis of 1-Cytosinyl-N-malayamycin A: A Potent Fungicide. Org. Process Res. Dev. 2006, 10 (3), 518-524. – Gram scale example of a potent Fungicide
Relevant scale-up example
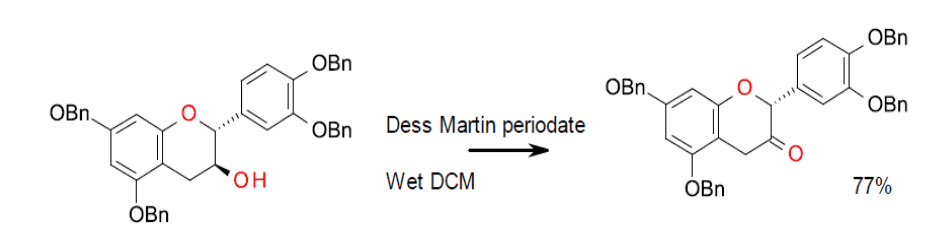
Experimental – Dess Martin periodate 10-L scale. Use of wet DCM gave a more predictable and faster reaction.
Sharma, P. K.; Kolchinski, A.; Shea, H. A.; Nair, J. J.; Gou, Y.; Romanczyk, L. J., Jr.; Schmitz, H.H. Scale-Up Syntheses of Two Naturally Occurring Procyanidins: (–)-Epicatechin-(4ß,8)-(+)-catechin and (–)-Epicatechin-3-O-galloyl-(4ß,8)-(–)-epicatechin-3-O-gallate. Org. Process Res. Dev. 2007, 11 (3), 422-430.
Green Review
- Atom efficiency (by-products)
Atom efficiency is very poor – removal of H2 gives 376 by-products. - Safety concerns
Dess Martin reagent , and intermediates can be explosive (J. Org. Chem. 1993, 58, 2899.) and should be handled appropriately. - Toxicity and environmental/aquatic impact
Organoiodine compounds, especially hydrophobic materials, present a moderate hazard to the aquatic environment and may bio accumulate. - Cost, availability & sustainable feedstocks
May have limited availability and transport issues for large scale use – very high cost reagent. - Sustainable implications
Incineration of waste streams could be problematic (iodine content). Limited utility for waste by-products. Iodine is an element at medium to high risk of depletion. A high Life Cycle Inventory (LCI) reagent, although it is possible to recover iodide from organoiodine waste materials. Preparation of the IBX reagent is a multi-step process and thus contributes considerable LCI burden.
Overall non-preferred to less atom intensive alternatives.