DMSO/DCC Pfitzner-Moffatt (also TFAA activation)
Mechanism + Description
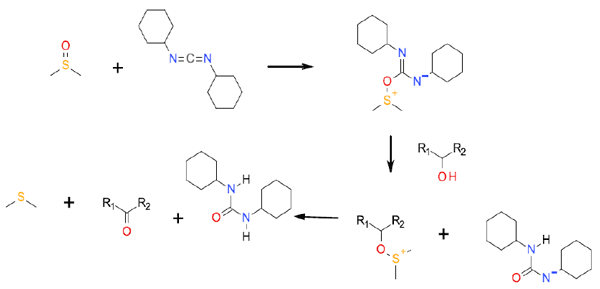
This Swern variant uses DCCI as the DMSO activator. The DMSO first attacks carbodiimide, which allows the added alcohol to attack the positively charged sulfur atom. Alpha hydro-elimination then results in oxidation to a carbonyl. Use of DCCI as an activator can increase the extent of Pummerer and elimination side reactions.
General comments
This variant is now largely replaced by trifluoroacetic anhydride and pyridine SO3. The relatively insoluble dicyclohexyl urea by-product can be problematic to remove, often precipitating from solution upon reaction completion, but also partially soluble enough to not be fully removed via filtration.
The dimethylsulfide by-product needs consideration due to stench and toxicity containment.
Key references
Pfitzner, K. E.; Moffatt, J. G. A New and Selective Oxidation of Alcohols. J. Am. Chem. Soc. 1963, 85 (19), 3027–3028.
Tidwell, T. T. Oxidation of Alcohols to Carbonyl Compounds via Alkoxysulfonium Ylides: The Moffatt, Swern, and Related Oxidations.
Organic Reactions. 1990, 39, 297–572.
Relevant scale-up example
No scale-up example with DCCI found.
TFAA activation variation of Pfitzner-Moffat
Mechanism + Description
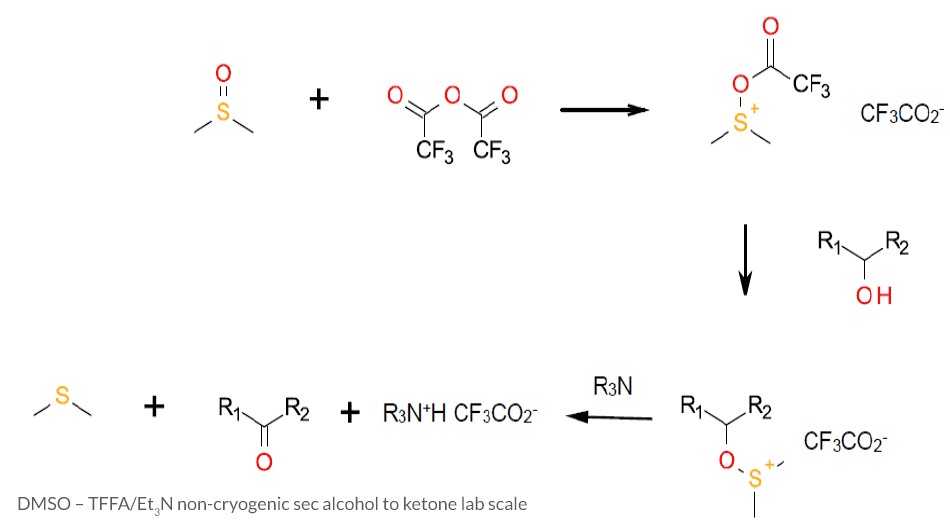
This Swern variant uses trifluoroacetic (TFAA) as the DMSO activator. DMSO first attacks TFAA, allowing the added alcohol to attack the sulfur atom. Alpha hydro-elimination results in oxidation to a carbonyl. In parallel with a continuous flow microreactor [3] it has been shown that Pummerer related by-products can be minimized.
Key references
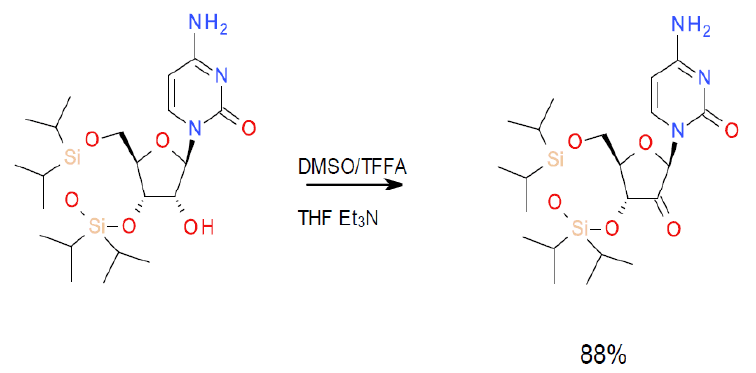
[1] Appell, R. B.; Duguid, R. J. New Synthesis of a Protected Ketonucleoside by a Non-Cryogenic Oxidation with TFAA/DMSO1. Org. Process Res. Dev. 2000, 4 (3), 172–174.
[2] Kawaguchi, T., Dr.; Miyata, H.; Ataka, K., Dr.; Mae, K.; Yoshida, J. Room-Temperature Swern Oxidations by Using a Microscale Flow System. Angewandte Chemie, International Edition. 2005, 44 (16), 2413-2416.
[3] van der Linden, J. J. M.; Hilberink, P. W.; Kronenburg, C. M. P.; Kemperman, G. J. Investigation of the Moffatt-Swern Oxidation in a Continuous Flow Microreactor System. Org. Process Res. Dev. 2008, 12 (5), 911–920.
Scale-up example
Green Review
- Atom efficiency (by-products)
Both activators have approximately the same molecular weight (DCCI, 206 g/mol and TFFA, 201 g/mol) and, therefore, comparable atom efficiency for the DSMO activator. The handling and waste disposal of each are quite different. Both need an organic base. - Safety concerns
The reaction produces Me2S gas and needs to be controlled. DCC requires strict containment. TFAA liberates 2 moles of TFA (strong corrosive acid). - Toxicity and environmental/aquatic impact
DCCI, dicyclohexyl carbodiimide, is a potent sensitizer. Me2S, while occurring naturally in the environment, is an irritant and volatile by-product with a disagreeable smell.
When using trifluoroacetic anhydride as an activator, the TFA by-product is persistent in the environment, and fluorinated materials can be problematic when considering incineration of wastes. - Cost, availability & sustainable feedstocks
DCC, TFAA, and DMSO are widely available and relatively cheap on scale. DCC is more commonly used for peptide couplings. - Sustainable implications
The DCC-urea by-product that forms upon work-up usually results in poor efficiencies that would best be removed at the start by reagent choice. Using TFAA as an activator allows for by-products to stay in solution and yields fewer complications.