DMSO –Oxalyl Chloride, Swern Oxidation
Mechanism + Description
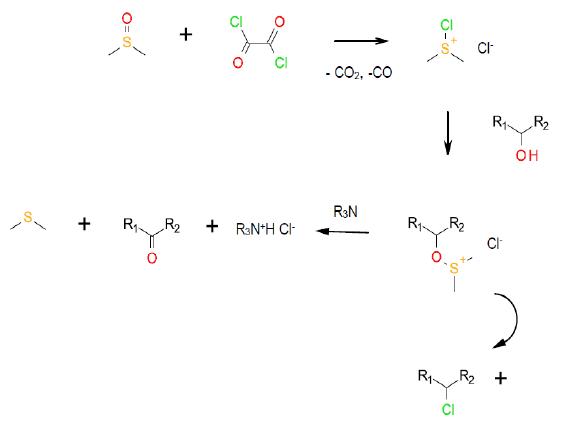 The Swern oxidation is one of a related series of oxidations based on activated DMSO. In the Swern varient, oxalyl chloride generates the dimethylchlorosulfonium chloride. Reaction with the alcohol produces an oxy sulphonium ion which undergoes base –catalyzed elimination to give the ketone and Me2S.
The Swern oxidation is one of a related series of oxidations based on activated DMSO. In the Swern varient, oxalyl chloride generates the dimethylchlorosulfonium chloride. Reaction with the alcohol produces an oxy sulphonium ion which undergoes base –catalyzed elimination to give the ketone and Me2S.
General comments
This non-catalytic oxidation that avoids heavy metals was once widely used. Drawbacks are that it needs cryogenic temperatures (-60 oC) and generates Me2S as a by-product. Other issues include dealing with exothermic chemistry and the potential for side reactions to occur if the temperature is not well-controlled. Other activators that give more controlled reactions and allow operation at higher temperatures have generally replaced oxalyl chloride.
The highly exothermic nature of Swern-type oxidations and the need for cryogenics to minimize side reactions make these oxidations good candidates for continuous flow reactions.
Key references
Omura, K.; Swern, D. Oxidation of alcohols “activated dimethyl sulfoxide. A preparative, steric and mechanistic study. Tetrahedron. 1978, 34 (11), 1651-1660.
Mancuso, A. J.; Brownfain, D. S.; Swern, D. Structure of the dimethyl sulfoxide-oxalyl chloride reaction product. Oxidation of heteroaromatic and diverse alcohols to carboynl compounds. J. Org. Chem. 1979, 44 (23), 4148–4150.
Russell McConnell, J.; Hitt, J. E.; Daugs, E. D.; Rey, T. A. The Swern Oxidation: Development of a High-Temperature Semicontinuous Process. Org. Process Res. Dev. 2008, 12 (5), 940-945.
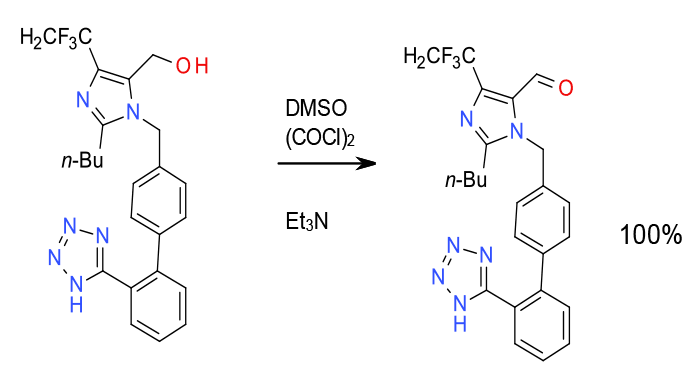
Relevant scale-up example
Green Review
- Atom efficiency (by-products)
Moderately atom efficient for an oxidation with 172 for loss of H2. - Safety concerns
CO2 and CO evolution from oxalyl chloride – very exothermic reaction and needs careful handling on scale. Me2S by-product needs to be controlled. - Toxicity and environmental/aquatic impact
Has similar effects to phosgene. - Cost, availability & sustainable feedstocks
Reagents are cheap and readily available, but the use of oxalyl chloride has now been largely phased out with other DMSO activators. - Sustainable implications
No large issues with reagents. Sustainability issues are usually dominated by solvents, normally chlorinated, used for the Swern oxidation.