DMSO – Pyridine-SO3 (Parikh-Doering)
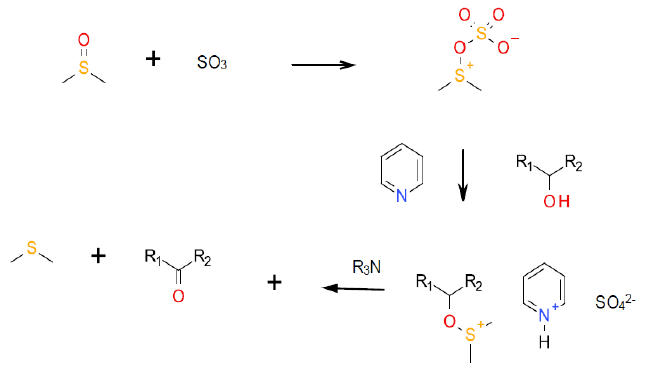 Mechanism + Description
Mechanism + Description
This widely used Swern variant uses pyridine-sulfur trioxide complex as an activating agent in a mechanism analogous to the Swern and Pfitzner-Moffat mechanisms.
General comments
These reagents are common in scale-up examples.
HSO4 is a common impurity in a commercial pyridine-SO3 complex. This can catalyze undesired side reactions, but can be effectively neutralized by the addition of extra pyridine to give 2 C5H5NH SO4.
Key references
Zanka, A.; Itoh, N.; Kuroda, S. Process Improvements in the Production of a Novel Non-Xanthine Adenosine A1 Receptor Antagonist. A “One-Pot” Horner-Emmons Isomerization Reaction. Org. Process Res. Dev. 1999, 3 (6), 394–399.
Waser, M.; Moher, E. D.; Borders, S. S. K.; Hansen, M. M.; Hoard, D. W.; Laurila, M. E.; LeTourneau, M. E.; Miller, R.D.; Phillips, M. L.; Sullivan, K. A.; et al. Process Development for a Key Synthetic Intermediate of LY2140023, a Clinical Candidate for the Treatment of Schizophrenia. Org. Process Res. Dev. 2011, 15 (6), 1266–1274.
Haycock-Lewandowski, S. J.; Wilder, A.; Åhman, J. Development of a Bulk Enabling Route to Maraviroc (UK-427,857), a CCR-5 Receptor Antagonist. Org. Process Res. Dev. 2008, 12 (6), 1094–1103.
Relevant scale-up example
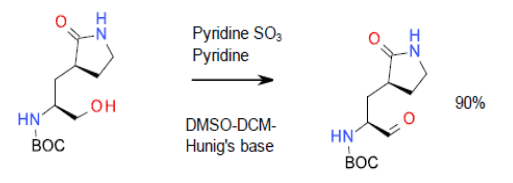
Org. Process Res. Dev. 2006, 10 (1), 163-164.
Green Review
- Atom efficiency (by-products)
Sulphur trioxide is oxidized to sulfate. Pyridine is recovered from the reaction unchanged, but never reused. The organic base is converted to the corresponding sulfate salt. - Safety concerns
This oxidation can be very exothermic. Me2S may off-gas from the reaction mixture. - Toxicity and environmental/aquatic impact
Pyridine is toxic to humans and the environment. SO3 is an acidic gas and contributes to acid rain. The DMS by-product occurs naturally in the environment, but it is an irritant, volatile, and has a disagreeable smell. - Cost, availability & sustainable feedstocks
Relatively cheap and available on-scale. Pyridine can also be purchased in polymer bound form, though at a cost premium. - Sustainable implications
Pyridine used to be made from coal tar and coal gasification, but greener routes from acetaldehyde and ammonia are now extensively used.