IBX 2-Iodoxybenzenesulfonic Acid
 Mechanism + Description
Mechanism + Description
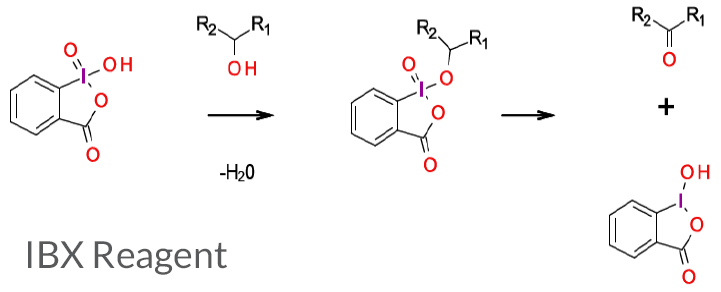
Condensation between an alcohol and IBX reagent with subsequent oxidation affords the carbonyl functionality (and reduction of the IBX).
General comments
2-iodoxybenzoic acid, IBX, is a hypervalent iodine (V) compound, often used as a versatile oxidant for the synthesis of aldehydes and ketones, as well as a variety of other functional group transformations. It has been widely adopted by medicinal chemists as a versatile and high yielding reagent that can be used under mild conditions with a wide variety of alcohols. While such oxidations have been scaled-up, this reagent suffers from poor atom economy and has been reported to be shock-sensitive as a pure solid. This has been attributed to contamination by strong oxidizing agents used in the synthesis. This material is often supplied stabilized with materials like benzoic and isophthalic acids. Variation in yields with different batches of the reagent has been observed. Stabilized versions are available and often referred to as “SIBX.”
Key references
Ozanne, A.; Pouységu, L.; Depernet, D.; François, B.; Quideau, S. A Stabilized Formulation of IBX (SIBX) for Safe Oxidation Reactions Including a New Oxidative Demethylation of Phenolic Methyl Aryl Ethers. Org. Lett. 2003, 5 (16), 2903-2906.
Duschek, A.; Kirsch, S. F. Ph. D. 2-Iodoxybenzoic Acid─A Simple Oxidant with a Dazzling Array of Potential Applications. Angewandte Chemie, International Edition. 2011, 50 (7), 1524-1552. (IBX analogue comparisons)
Tanaka, Y.; Fuse, S.; Tanaka, H.; Doi, T.; Takahashi, T. An Efficient Synthesis of a Cyclic Ether Key Intermediate for 9-Membered Masked Enediyne Using an Automated Synthesizer. Org. Process Res. Dev. 2009, 13 (6), pp 1111–1121. (scale up example)
Thottumkara, A. P.; Bowsher, M. S.; Vinod, T. K. In Situ Generation of o-Iodoxybenzoic Acid (IBX) and the Catalytic Use of It in Oxidation Reactions in the Presence of Oxone as a Co-oxidant. Org. Lett. 2005, 7 (14), 2933-2936. (in-situ generation)
Relevant scale-up examples
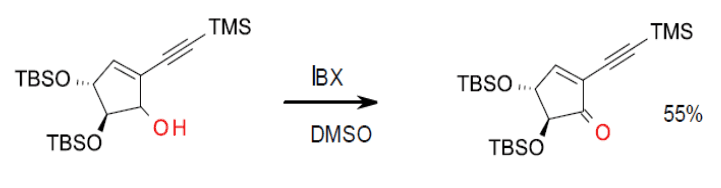
Org. Process Res. Dev. 2009, 13 (6), 1111–1121.
Green Review
- Atom efficiency (by-products)
Unless catalytic, very poor – removal of H2 gives 264 by-product - Safety concerns
Un-stabilized IBX is a potential explosive. Numerous studies into stabilization, catalytic cycles or viable (analogous) alternatives – if needed, catalytic options are better. - Toxicity and environmental/aquatic impact
Organoiodine compounds, especially hydrophobic materials, present a moderate hazard to the aquatic environment and may bio accumulate. IBX is a severe irritant and causes burns. - Cost, availability & sustainable feedstocks
May have limited availability and transport issues for large scale use – very high cost reagent - Sustainable implications
Incineration of waste streams could be problematic (iodine content). There is limited utility for waste by-products. Iodine is an element at medium to high risk of depletion (High LCA reagent), although it is possible to recover iodide from organoiodine waste materials. Preparation of the IBX reagent is a multi-step process and thus contributes considerable Life Cycle Inventory (LCI) burden. This reagent is overall non-preferred to less atom intensive alternatives.
Catalytic variations
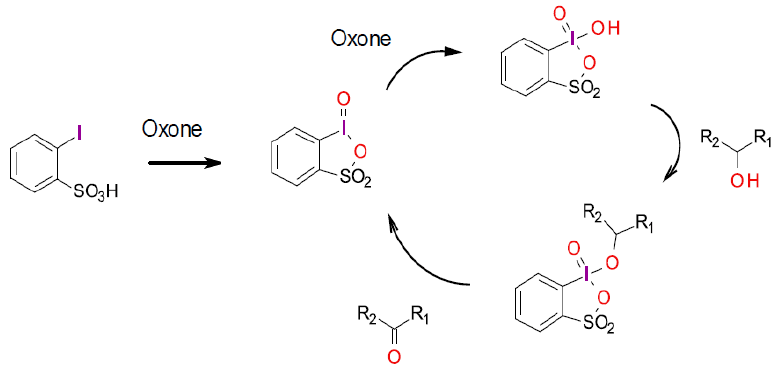 Increasingly, stoichiometric IBX oxidations are being replaced by catalytic versions using a small amount of the hypervalent iodine reagent generated in situ, driven by an added safer, more environmentally acceptable terminal oxidant.
Increasingly, stoichiometric IBX oxidations are being replaced by catalytic versions using a small amount of the hypervalent iodine reagent generated in situ, driven by an added safer, more environmentally acceptable terminal oxidant.
References to catalytic IBX and its variants
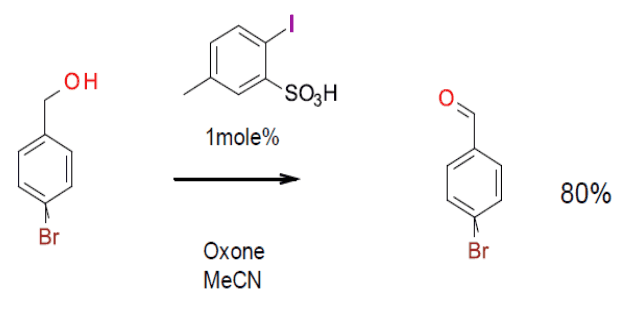
Uyanik, M.; Akakura, M.; Ishihara, K. 2-Iodoxybenzesulfonic Acid as an Extremely Active Catalyst for the Selective Oxidation of Alcohols to Aldehydes, Ketones, Carboxylic Acids, and Enones with Oxone. J. Am. Chem. Soc. 2009, 131 (1), 251-262. (IBX with additional EDG’s, and iodobenzenesulfonic acid –IBS examples)
Thottumkara, A. P.; Bowsher, M. S.; Vinod, T.K. In Situ Generation of o-Iodoxybenzoic Acid (IBX) and the Catalytic Use of It in Oxidation Reactions in the Presence of Oxone as a Co-oxidant. Org. Lett. 2005, 7 (14), 2933-2936. (catalytic generation of IBX using oxone)
Uyanik, M.; Ishihara, K. 2-Iodoxy-5-Methylbenzenesulfonic Acid-Catalyzed Selective Oxidation of 4-Bromobenzyl Alcohol to 4-Bromobenzaldehyde or 4-Bromobenzoic Acid with Oxone. Org. Synth. 2012, 89, 105-114. (Experimental for catalytic IBS oxidation – gram scale)