Manganese Dioxide, MnO2
Mechanism + Description
The mechanism is unclear. The reaction is heterogeneous and believed to occur via a radical mechanism on the surface of the MnO2 particles, which is why activated alcohols are the usual substrates for MnO2, and simple unactivated alcohols react at a very slow rate.
General comments
Manganese dioxide is a traditional oxidant used for functional group oxidations in synthesis. Often reactions are heterogeneous and require a large molar excess (often 10 x or more) of MnO2 to drive to completion. Yield and reproducibility are reported to be very variable with the quality and age of the reagent along with the acidity of the reaction (which can be affected by aging reagents), which is why many papers recommend using freshly prepared MnO2. Waste MnO2 can be recycled to permanganate. The rate of oxidation of unactivated alcohols with MnO2 is often too slow to be practically useful for synthesis.
Key references
MnO2 waste recycled to MnO4– , Org. Process Res. Dev., 2001, 5 (6), pp 599–603
Solvent free oxidation of alcohols with manganese dioxide, Tetrahedron Letters, 2002, 43(35), 6149-6150
Active Manganese dioxide oxidation in organic chemistry. Part I, Synthesis, 1976, (2), 65-104.
Relevant scale up examples with Scheme
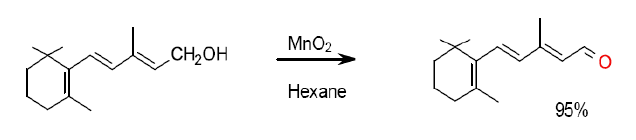
Org. Process Res. Dev., 2005, 9 (3), pp 302–305
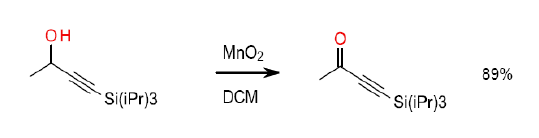
Org. Synth. 2007, 84, 120
Green Review
- Atom efficiency
(by-products Mwt) Stoichiometric atom efficiency gives MnO and H2O as by-products, but large excess of MnO2 needed leads to very poor mass economy – so overall poor - Safety Concerns
No issues - Toxicity and environmental/aquatic impact
Mn is an essential element, but toxic in higher doses - Cost, availability & sustainable feedstocks
MnO2 and precursor KMnO4 are readily available and cheap. If MnO2 has to be prepared, solvent and energy use to do this needs to be considered. Use of MnO2 may incur high solvent use during product isolation and plant cleaning. Can be recycled - Sustainable implications
Despite excess MnO2 used, it can and should be recycled otherwise disposal is via land fill. All metals have a high LCA impact from mining and refining operations, so use should be catalytic with recovery and recycle. Mn is listed as at moderate risk of depletion from natural resources