NaOCl Bleach Oxidation
Mechanism + Description
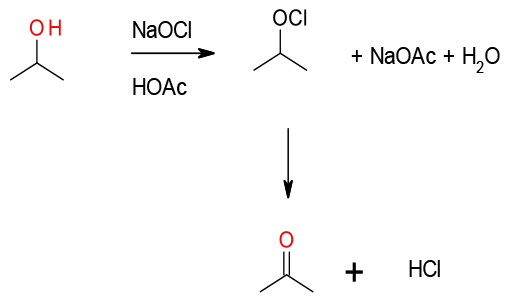 A non-catalytic, non- metal catalysed oxidation mechanistically related to the Swern oxidation, albeit with N-Chlorosuccinimide
A non-catalytic, non- metal catalysed oxidation mechanistically related to the Swern oxidation, albeit with N-Chlorosuccinimide
Activating the DMS to attack from the alcohol. Chlorinated by-products can result in some instances.
General comments
NaOCl is widely used as a terminal oxidant with metal and nitroxyl radical catalysts, bleach alone will oxidise hydroxyl compounds but this is very dependent on reaction conditions used, especially solvent. There are several reports of the synthesis of ketones from secondary alcohols. NaOCl in acetic acid is a cheap and efficient reagent for the oxidation of secondary alcohols to ketones.
Key references
J. Org. Chem., Vol. 45, No. 10, 1980 2031 – Convenient and Inexpensive Procedure for Oxidn of 2nd alcohols to Ketones
Org. Process Res. Dev., 2012, 16 (5), pp 1082–1089 – Continuous Flow Oxidation of Alcohols and Aldehydes Utilizing Bleach and Catalytic Tetrabutylammonium Bromide
Journal of Fluorine Chemistry (2012), 140, 95-98 – Bleach, metal free oxidation in fluorinated solvents
Relevant scale up example
No scale up examples identified
Green Review
- Atom efficiency (by-products Mwt)
Good – NaCl and water as by-products of the oxidation. - Safety Concerns
Potential for very exothermic reactions. Bleach may react violently with certain classes of compounds – reductants, amines etc. - Toxicity and environmental/aquatic impact
Generally low hazard if the excess reagent is neutralised before disposal. As with all positive halogen sources, there is some concern over the potential production of low levels of polyhalogenated endocrine disrupting compounds in the environment. - Cost, availability & sustainable feedstocks
Readily available and very cheap. - Sustainable implications
Discarding oxygen, bleach is one of the cheapest and cleanest oxidizing reagents.