Nickel Peroxide, NiO(OH)2
(includes in-situ preparation from other Nickel salts)
Mechanism + Description
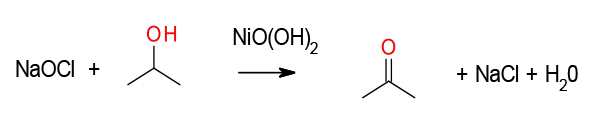 The mechanism is unclear – evidence suggests radical rather than ionic. Nickel oxide hydroxide – NiO(OH)2 appears to act as a heterogeneous catalyst commonly generated in-situ from Nickel salts such as NiCl2 or Ni(OAc)2 which results in high surface area nanoparticles (ca. 4 nm).
The mechanism is unclear – evidence suggests radical rather than ionic. Nickel oxide hydroxide – NiO(OH)2 appears to act as a heterogeneous catalyst commonly generated in-situ from Nickel salts such as NiCl2 or Ni(OAc)2 which results in high surface area nanoparticles (ca. 4 nm).
General comments
Nickel peroxide is commonly termed, though its probably NiO(OH)2). The actual active nickel substrate was originally used in molar excess, however recently catalytic variants are more routine and are more attractive to run on scale. The oxidn. of primary alcohols to carboxylic acids, secondary alcs. to ketones, aldehydes to carboxylic acids, and α,β-unsatd. carboxylic acids to epoxy acids is demonstrated using 2.5 mol % of nickel catalyst and common bleach as the terminal oxidant.
2.5 mol % of NiCl2, 45 mmol alcohol, 15 mL of CH2Cl2, 300 mL of 5% aq. NaOCl, 2 h at 0 °C, then 2 h at 20 °C. organic solvent-free conditions: same, except no CH2Cl2. J. Org. Chem, Vol. 71, No. 25, 2006 9293
Key references
- An Efficient and Practical System for the Catalytic Oxidation of Alcohols, Aldehydes, and α, β-Unsaturated carboxylic acids, Journal of Organic Chemistry, 2006, 71(25), 9291-9296.
- Mild and efficient oxidation of primary and secondary alcohols using NiO2/silica gel system (solvent free), E-Journal of Chemistry, 2011, 8(2), 491-494.
- Nickel peroxide oxidation of organic compounds, Chemical Reviews, 1975, 75(4), 491-519.
Relevant scale up examples with Scheme
No examples found
Green Review
- Atom efficiency (by-products Mwt)
Good catalytic efficiency; by-products are water and inorganic salts NaCl – overall good - Safety Concerns
Major operational safety issues are with use of reoxidants like bleach and peroxide with organic materials. Should consider dose control for large scale reactions. Radical mechanism might highlight incompatibility with functional groups liable to polymerization. - Toxicity and environmental/aquatic impact
High Ni concentrations in the environment can damage plant life. Some Ni salts are sensitizers and known or suspected carcinogens. - Cost, availability & sustainable feedstocks
Ni compounds are very cheap as are re-oxidants to complete the catalytic cycle. Little scale-up information has been located using this reagent. Metal can be recycled, but low cost of virgin material may make this economically unattractive. - Sustainable implications
Some Ni compounds are on the European Union REACH Substances of Very High Concern (SVHC) list. Ni is a very abundant metal and is currently not rated at risk of depletion. All metals have a high LCA impact from mining and refining operations, so use should be catalytic with recovery and recycle.
| Classification | Oral Exposure | Parenteral Exposure | Inhalation exposure* | ||
| PDE (μg/day) |
Concentration (ppm) |
PDE (μg/day) |
Concentration (ppm) |
PDE (ng/day) |
|
| Class 1A: Pt, Pd Class 1B: Ir, Rh, Ru, Os Class 1C: Mo, Ni, Cr, V Metals of significant safety concern |
100 100** 250 |
10 10** 25 |
10 10** 25 |
1 1** 2.5 |
Pt: 70* Ni: 100 Cr(VI): 10 |
| Class 2: Cu, Mn Metals with low safety concern |
2500 | 250 | 250 | 25 | |
| Class 3: Fe, Zu Metals with minimal safety concern |
13000 | 1300 | 1300 | 130 | |
Class Exposure and Concentration Limits for Individual Metal Catalysts and Metal Reagents in pharmaceuticals – EMEA guidance