Oppenauer Oxidation: An Integrated Approach
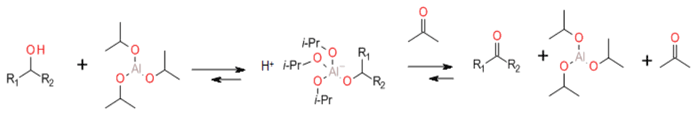
The reaction mechanism of Meerwein-Ponndorf-Verley-Oppenauer (MPVO) reactions proceeds in most cases via a cyclic six-membered transition state in which both reductant and oxidant are coordinated to the metal centre of a metal alkoxide catalyst.
General comments
This reaction first published by Oppenauer in 1937 can have a reputation as an old fashioned method that has been superseded by more modern reagents. But the reaction is very green just using catalytic amounts of Al(OtBu)3 or Al(OiPr)3 as base with isopropanol the only by-product. The reaction is also commonly used on a large scale. It is hoped that the increased focus on Green synthetic methods will bring about a rebirth of interest in the Oppenauer oxidation. The reaction can be driven in oxidation or reduction mode using simple alcohols or ketones as donors or acceptors. Other Al3+ complexes can be employed as catalysts, and other metals like Fe3+ can be used. In the presence of chiral ligands, enantioselective oxidation can occur.
Key references
Synthesis 1994; 1994(10): 1007-1017 – Integrated approach to Meerwein-Ponndorf-Verley reductions and Oppenauer oxidations (MPVO)
Journal of the American Chemical Society (2006), 128(39), 12596-12597– Selective Al-catalysed Oppenauer oxidations
Org. Process Res. Dev., 2012, 16 (7), pp 1301–1306 – Al(OtBu)3 as an effective catalyst
Relevant scale up example
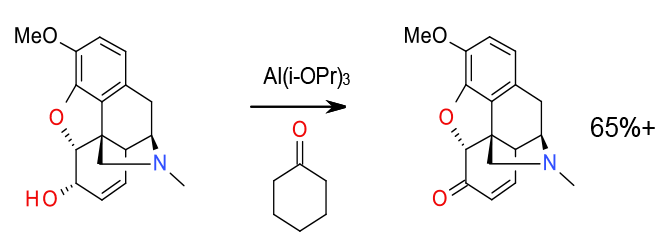
Chiu, F.-T.; Lo, Y. S. U.S. Patent 6,177,567, 2001
Chem. Rev., 2006, 106 (7), pp 2943–2989
Green Review
- Atom efficiency (by-products Mwt)
Atom efficiency depends on sacrificial ketone used and the number of equivalents needed – AE can be good provided a large excess of acceptor is not needed to drive the equilibrium. - Safety Concerns
No major concerns. - Toxicity and environmental/aquatic impact
Aluminium wastes are manageable, especially if used in catalytic quantities. - Cost, availability & sustainable feedstocks
Materials readily available and cheap. - Sustainable implications
All metals have a high LCA impact from mining and refining operations, so use should be catalytic with recovery and recycle. Aluminum is currently listed as being at low risk of depletion. When used catalytically, recycling may not be economically feasible.