Oxidation with Chlorine /Pyridine Complexes
Mechanism + Description
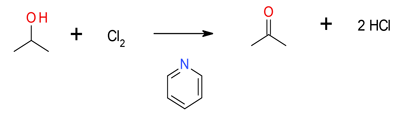 Mechanism is not clear – the active oxidant is probably the charge transfer complex formed between pyridine and chlorine or the ionic salt C6H5NCl+ Cl–
Mechanism is not clear – the active oxidant is probably the charge transfer complex formed between pyridine and chlorine or the ionic salt C6H5NCl+ Cl–
General comments
Mixtures of Cl2 and pyridine have been reported to oxidize alcohols to carbonyl compounds – as have other halogens. These reactions can selectively oxidize secondary over primary alcohols. This is not a widely adopted reaction. Cl2 and pyridine both have toxicity and occupational exposure issues associated with their use. Environmental releases are also strictly controlled.
Key references
Tetrahedron Letters (1974), (35), 3059-62 – selective oxidation of secondary alcohols
Tetrahedron (2001), 57(49), 9765-9788 – selective oxidation of secondary alcohols
Relevant scale up example
No scale up examples identified