PCC Review on Cr(VI) oxidation – Corey’s Reagent
Mechanism + Description
Most Chromate (VI) reagents work via a common mechanism – formation of a chromate ester followed by oxidative elimination of Cr (IV) and the oxidised alcohol product.
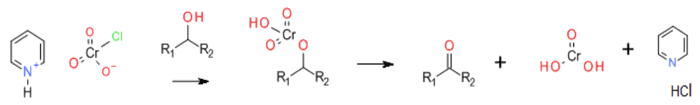
General comments
PDC and the related PCC are among a range of Cr (VI) oxidants. Other variants exist with modifications like amine ligands etc. Once widely used, there are still recent publications claiming greener and solvent free oxidations with Cr reagents – this said, chromium reagents should almost always be viewed as a less preferred option in terms of green chemistry.
An improved procedure for the preparation of Corey’s reagent—Pyridinium chlorochromate has been described. The method is less hazardous and gives better yield. Synthetic utility of the reagent has been shown to increase in the presence of anhydrous acetic acid, used for the first time as catalyst, for the oxidation of alcohols.
Key references
Relevant scale up example
No example found
Green Review
- Atom efficiency (by-products Mwt)
Removal of H2 generates 218 mass of waste including pyridine HCl and Cr(III) oxides - Safety Concerns
Chromium reagents strongly support combustion and can present an explosion hazard in contact with organic material. - Toxicity and environmental/aquatic impact
Chromium (VI) compounds are both acutely toxic (irritant) and known to be carcinogenic to mammals, and are also very hazardous to aquatic organisms. Many Cr (VI) compounds are on the European union REACH Substances of Very High Concern (SVHC) list due to their carcinogenic properties. - Cost, availability & sustainable feedstocks
Chromium reagents are cheap and readily available although increasingly stringent legislation may make their use increasing difficult.
http://echa.europa.eu/candidate-list-table
http://www.epa.gov/ttnatw01/hlthef/chromium.html - Sustainable implications
These reagents often form intractable residues after reduction that can result in protracted cleaning procedures. All metals have a high LCA impact from mining and refining operations, so use should be catalytic with recovery and recycle. Chromium is currently listed as being at low risk of depletion.