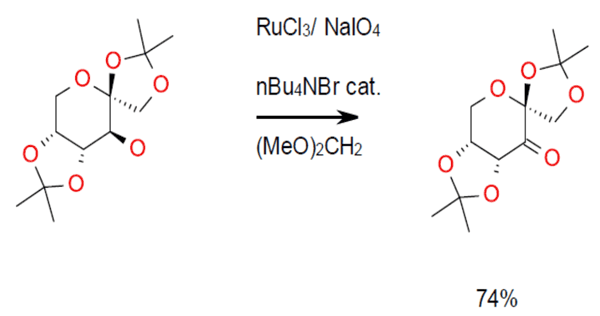
RuCl3
Mechanism + Description
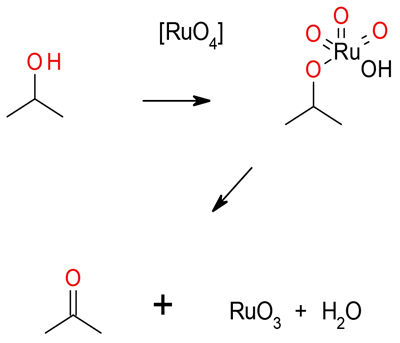 Ru (III) salts are oxidised in situ by a terminal oxidant
like NaIO4, NaOCl, H2O2 to RuO4, which is the active
species. Oxidation is via Ru ester, the reduced Ru(VI)
species is recycled back to RuO4 by the oxidant
Ru (III) salts are oxidised in situ by a terminal oxidant
like NaIO4, NaOCl, H2O2 to RuO4, which is the active
species. Oxidation is via Ru ester, the reduced Ru(VI)
species is recycled back to RuO4 by the oxidant
General comments
RuCl3 can be used catalytically with a terminal oxidant added to drive the reaction Generally Ruthenium salts are low impact with low catalytic loadings. As with all heavy metals, contamination of the environment needs to be avoided and levels in the final API need to be controlled. As with all precious metals, use of more abundant alternatives should be evaluated as a priority. Other risks depend on the co-oxidant used.
Key references
Org. Proc. Res. Dev., 2004, 8 (6), pp 931–938 – Use with TBAB and TCC to oxidise alcohols
J. Org. Chem., 1988, 53 (15), pp 3553–3555 Use with H2O2 and PTC conditions