TCA Trichloroisocyanuric Acid: A Safe and Efficient Oxidant
Mechanism + Description
Probably via deprotonation and hydride abstraction. In the presence of pyridine, pyridinium N-chloride is part of the active oxidant. All three active chlorine atoms are available for oxidation.
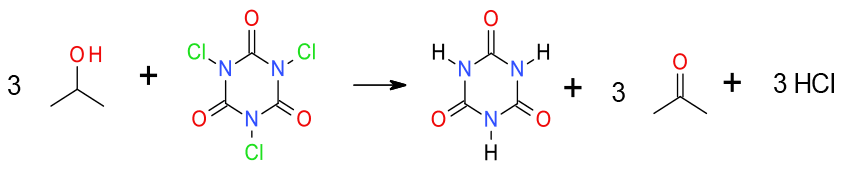
General comments
As with bleach, under certain conditions, TCCA will oxidise alcohols to carbonyl compounds in the absence of an added catalyst. TCCA often shows good selectivity for the oxidation of secondary alcohols in the presence of primary alcohols.
Generally TCCA is a safe and efficient reagent, useful for chlorination and oxidation even on large scale. It can oxidise alcohols to aldehydes and ketones in absence of TEMPO. A recent publication from DSM has reported that pyridine, usually added as a base, is much more effective as a catalyst in the presence of an inorganic base. Coupling as a reoxidant with catalysts such as RuCl3 and TEMPO can prove effective.
Key references
Syn. Commun. 1992,22, 1589 The Oxidation of Secondary Alcohols to Ketones with Trichloroisocyanuric Acid
Org. Proc. Res. Dev., 2002, 384–393 Trichloroisocyanuric Acid: A Safe and Efficient Oxidant
Catal. Sci. Technol., 2012, 2, 2052–2056 – trichloroisocyanuric acid & pyridine, from base to organocatalyst
Org. Lett., 2001, 3 3041–3043 A Very Mild and Chemoselective Oxidation of Alcohols to Carbonyl Compounds
Relevant scale up example

Org. Process Res. Dev., 2002, 6 (4), pp 384–393
Green Review
- Atom efficiency (by-products Mwt)
Atom economy is good producing 3 moles of HCl and 1 mole of cyanuric acid for 3 moles ketone/aldehyde. - Safety Concerns
All N-chloro materials are high energy compounds and can decompose violently in the presence of certain materials or if heated. Reacts with water to generate highly acidic solutions. Oxidations with TCA can be very exothermic. - Toxicity and environmental/aquatic impact
Generally low hazard if the excess reagent is neutralised before disposal. Cyanuric acid is classified as non-toxic. - Cost, availability & sustainable feedstocks
This reagent is cheap and available on scale. It is produced as a disinfectant for water- swimming pools. - Sustainable implications
Produced by chlorination of cyanuric acid with Cl2. Cyanuric acid is in turn made from pyrolysis of urea.