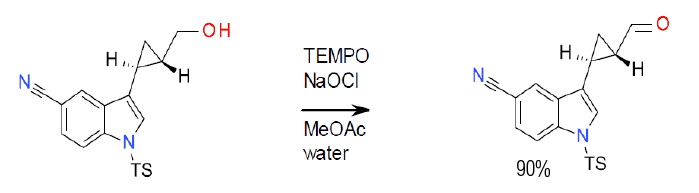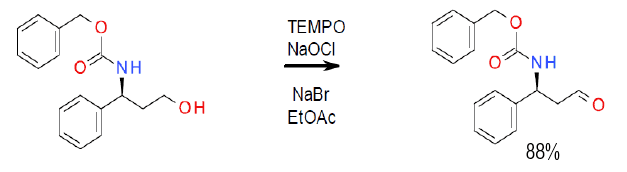TEMPO-Bleach Oxidation
Mechanism + Description
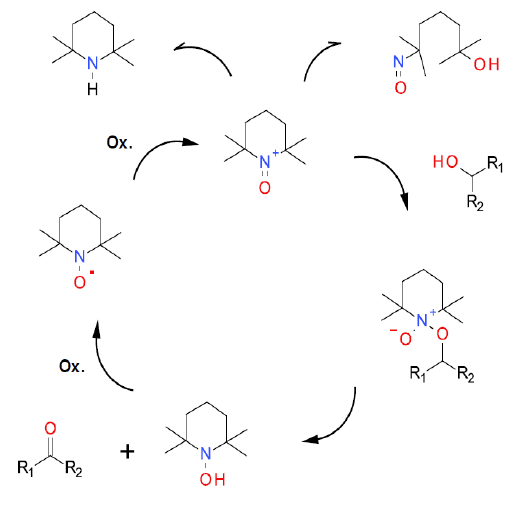 See “TEMPO (General overview).”
See “TEMPO (General overview).”
NaOCl is often used as a co-oxidant, generating NaCl as a by-product. NaBr or borates are often added as a promoter.
General comments
A common terminal oxidant is bleach (NaOCl), which is often employed with a bromide or borate co-catalyst. Bi-phasic reactions in water are often helped by the addition of a phase transfer catalyst.
Key references
Fritz-Langhals, E. Production of Aldehydes by Continuous Bleach Oxidation of Alcohols Catalyzed by 4-Hydroxy-TEMPO. Org. Process Res. Dev. 2005, 9 (5), 577–582.
Rossi, F.; Corcella, F.; Saverio Caldarelli, F.; Heidempergher, F.; Marchionni, C.; Auguadro, M.; Cattaneo, M.; Ceriani, L.; Visentin, G.; Ventrella, G.; et al. Process Research and Development and Scale-up of a 4,4-Difluoro-3,3-dimethylproline Derivative. Org. Process Res. Dev. 2008, 12 (2), 322-338. – Discussion of optimisation to prevent racemisation (50 L scale)
Hobson, L. A.; Akiti, O.; Deshmukh, S. S.; Harper, S.; Katipally, K.; Lai, C. J.; Livingston, R. C.; Lo, E.; Miller, M. M.; Ramakrishnan, S.; et al. Development of a Scaleable Process for the Synthesis of a Next-Generation Statin. Org. Process Res. Dev. 2010, 14 (2), 441-458. – DOE and robustness studies on TEMPO stage statin oxd’n (2000 L scale)
Webel, M.; Palmer, A. M.; Scheufler, C.; Haag, D.; Müller, B. Development of an Efficient Process Towards the Benzimidazole BYK308944: A Key Intermediate in the Synthesis of a Potassium-Competitive Acid Blocker. Org. Process Res. Dev. 2010, 14 (1), 142–151. – Use of NaI to prevent chlorination of heteroaromatic (50 L scale)
Relevant scale-up example
Green Review
- Atom efficiency (by-products)
Generally good –the removal of H2 generates NaCl (58) as a by-product - Safety concerns
All TEMPO oxidations are exothermic and may present delayed exotherms. Compatibility of NaOCl with other reaction components must be considered. - Toxicity and environmental/aquatic impact
Generally low when used catalytically, the major concerns arise from the co-oxidant. Nitroxyl radicals like TEMPO and the hydroxylamine intermediates in the oxidation cycle give positive structural alerts as potential genotoxic impurities (PGI). - Cost, availability & sustainable feedstocks
The cost of TEMPO has fallen over time, and it is now available in bulk. Other analogs are less commercially available and much more expensive, but sometimes display far greater activity. The skeleton of TEMPO comes from acetone and ammonia. - Sustainable implications
With optimization of catalyst loading and a low molecular weight terminal oxidant like NaOCl, this oxidation is a good choice. The major concern would be the solvent used. Many initial publications used dichloromethane, but later work has shown more sustainable solvents can be used (see references).