TEMPO (General Overview)
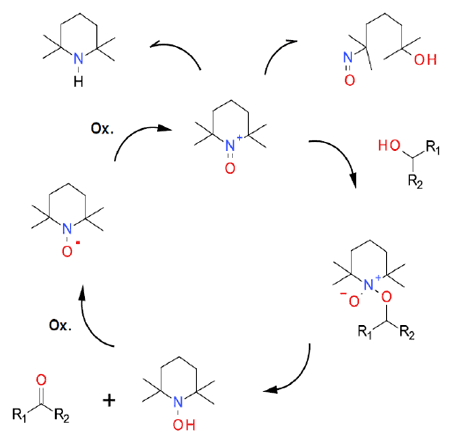 Mechanism + Description
Mechanism + Description
Several stable oxonium salts have been reported to be efficient oxidants in the synthesis of aldehydes and ketones from alcohols. These show very poor atom economy, but can be made catalytic with the active oxonium ion generated in situ with a terminal oxidant.
General comments
TEMPO and related stabilized nitroxyl radicals have found wide application as catalysts in green oxidation technology for the synthesis of aldehydes and ketones. The actual oxidant, the oxonium ion, can be used in stoichiometric amounts, but this adds cost and poor atom economy. Most are used catalytically with the active oxidant generated in situ and with the process driven by the addition of a terminal oxidant. Achieving very low catalyst loadings can be hindered by side reactions that destroy the oxidized form of the catalyst. Many of the early examples of TEMPO–catalysed oxidations were run in chlorinated solvents. More recent work has shown that greener solvents can be used.
Key references
Strohmeyer, H. E.; Sluggett, G. W. Determination and control of TEMPO, a potentially genotoxic free radical reagent used in the synthesis of filibuvir. Journal of Pharmaceutical and Biomedical Analysis. 2012, 62, 216-219.
Janssen, M. H. A.; Chesa Castellana, J. F.; Jackman, H.; Dunn, P. J.; Sheldon, R. A. Towards greener solvents for the bleach oxidation of alcohols catalysed by stable N-oxy radicals. Green Chem. 2011, 13, 905-912.
Ciriminna, R.; Pagliaro, M. Industrial Oxidations with Organocatalyst TEMPO and Its Derivatives. Org. Process Res. Dev. 2010, 14 (1), 245-251.
Relevant scale-up example
As stoichiometric use is generally not progressed, examples will be provided with individual oxidants in following slides.
Environmental considerations
Generally low impact when used catalytically, the major concerns arise from the choice of co-oxidant. Air and NaOCl give good atom economy, less so with hypervalent iodine oxidants which produce iodobenzene as a co-product. Nitroxyl radicals like TEMPO and the hydroxylamine intermediates in the oxidation cycle give positive structural alerts as potential genotoxic impurities (PGI).
Other TEMPO-related useful references
Anelli, P. L.; Biffi, C.; Montanari, F.; Quinci, S. Fast and selective oxidation of primary alcohols to aldehydes or to carboxylic acids and of secondary alcohols to ketones mediated by oxoammonium salts under two-phase conditions. J. Org. Chem. 1987, 52 (12), 2559-2562.
– Use of two-phase systems and phase transfer catalysis to increase kinetics of TEMPO reactions
Kuang, Y.; Rokubuichi, H.; Nabae, Y.; Hayakawa, T.; Kakimoto, M. A Nitric Acid-Assisted Carbon-Catalyzed Oxidation System with Nitroxide Radical Cocatalysts as an Efficient and Green Protocol for Selective Aerobic Oxidation of Alcohols. Advanced Synthesis & Catalysis. 2010, 352(14+15), 2635-2642.
– Nitric acid and carbon – catalyzed oxidation, similarity to TEMPO nitroxide radical catalyst
Siedlecka, R.; Skarzwewski; Młochowski, J. Selective oxidation of primary hydroxy groups in primary-secondary diols. Tetrahedron Lett. 1990, 31, 2177-2180.
– Selective TEMPO primary oxidation when primary and secondary alcohols present
Hampton, P. D.; Whealon, M. D.; Roberts, L. M.; Yaeger, A. A.; Boydson, R. Continuous Organic Synthesis in a Spinning Tube-in-Tube Reactor: TEMPO-Catalyzed Oxidation of Alcohols by Hypochlorite. Org. Process Res. Dev. 2008, 12 (5), 946–949.
– Spinning tube (continuous processing) TEMPO catalyzed oxidation