Aldolase
Mechanism + Description
There are a number of different classes of aldolase, some are metal dependant (Zn2+) in the active site and some work through a lysine in the active site. They couple aldehydes and ketones typically via conversion of one coupling partner from an electrophile to a nucleophilic imine/enamine followed by the normal aldol process to make the C-C bond. The enantioselectivity is controlled by the chiral environment of the active site. Scheme shows 2-Deoxy-d-ribose-5-phosphate aldolase (DERA), a class I aldolase, coupling acetaldehyde and chloroacetaldehyde.

General comments
There are a range of aldolase enzymes used to make chiral carbon-carbon bonds. Some are very substrate specific and require phosphorylated molecules for binding in the active site, so are not that attractive as catalysts to make a wide range of unnatural substrates at scale. Of greatest interest at this time are DERA-type aldolases and threonine aldolases that will accept a reasonable range of non-phosphorylated substrates.
Key references
Relevant scale up examples
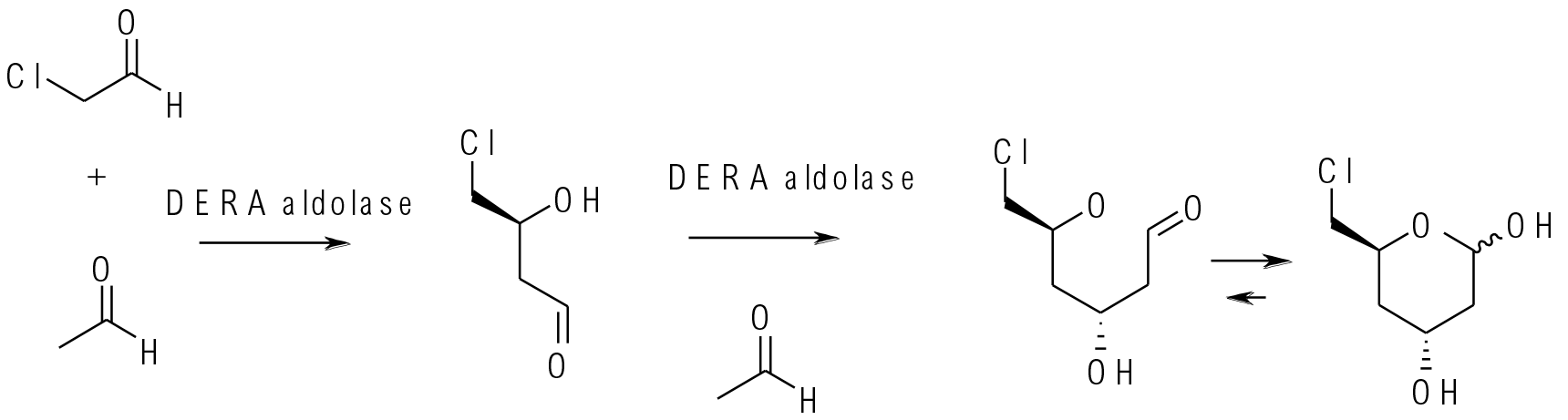
Biotechnol J. 2006, 1 (5), 537-548
100s kg scale

Org. Process Res. Dev. 2013, 17, 854-862
20 g scale
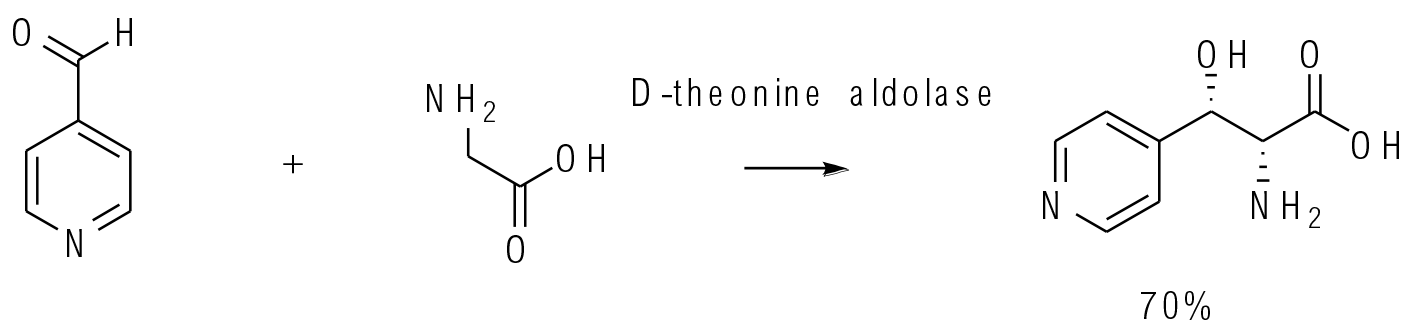
Org. Process Res. Dev. 2015, 19, 1317-1322
200 g scale
Green Review
-
Atom efficiency (by-products, molecular weight)
Catalytic technology—but enzyme loading should be optimized and steps using stoichiometric reagents like cofactor recycle should be optimized to avoid unnecessary excess of reagents. - Safety Concerns
Generally considered a safe technology to scale-up. Issues—enzymes/proteins can be sensitizers by inhalation, and some by mode of action can be skin irritants (proteases). If viable GMO cells are used, local regulations relating to use of GMOs need to be followed. - Toxicity and environmental/aquatic impact
No real concerns—enzymes are non-toxic and readily biodegradable. If viable GMO cells are used, these need to be passivated before discharge into the environment. - Cost, availability & sustainable feedstocks
Enzymes are produced from natural sustainable feedstocks. - Sustainable implications
Enzymes are made via fermentation; cloned and recombinant enzymes would be at no risk from depletion. Enzymes break down in the environment and the constituent amino acids are recycled in nature. The use of over expressed recombinant enzymes is generally much better than natural enzymes on a life cycle impact basis.