P450 Enzymes
Mechanism + Description
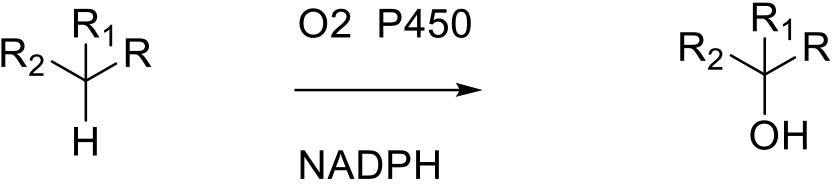
The active site of cytochrome P450 contains a heme-iron complex. The iron is bound to the protein via a cysteine thiolate ligand. The iron is oxidized by O2 to a highly reactive iron(IV) oxo (or ferryl) species which oxidises the substrate via a radical mechanism. The heme portion is connected to other domains flavin adenine dinucleotide / iron-sulfur ferredoxin and flavin mononucleotide to complete the electron transport process.
General comments
P450 enzymes are used for various oxidation reactions—monohydroxylation, oxidation of hetero atoms, epoxidation, oxidative dealkylation and other oxidations such as aromatization. Mainly of interest for the synthesis of metabolites and late-stage functionalization, p450 reactions can be difficult to scale using traditional mammalian like p450s that are insoluble and membrane bound. Self-sufficient p450s in which the redox domains are linked to each other and are soluble, e.g., BM3 type p450s, show more promise in scaled processes.
Key references
Fasan, R. Tuning P450 Enzymes as Oxidation Catalysts. ACS Catal. 2012, 2, 647–666.
Relevant scale up examples
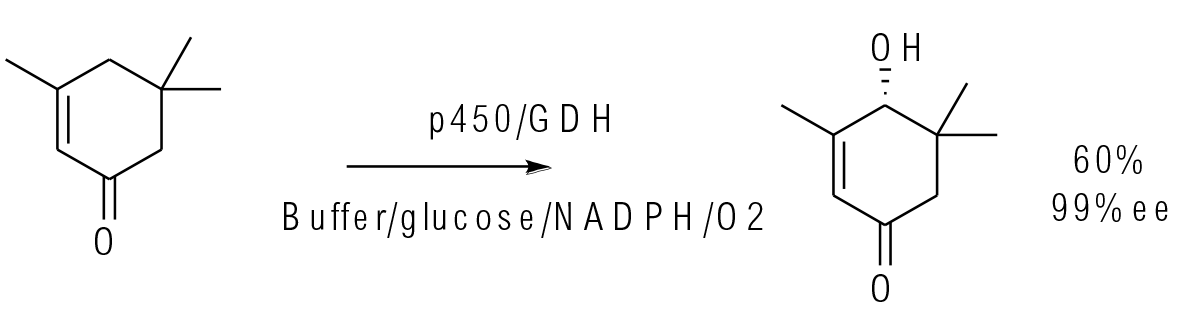
Org. Process Res. Dev. 2016, 20, 814−819
1 kg scale
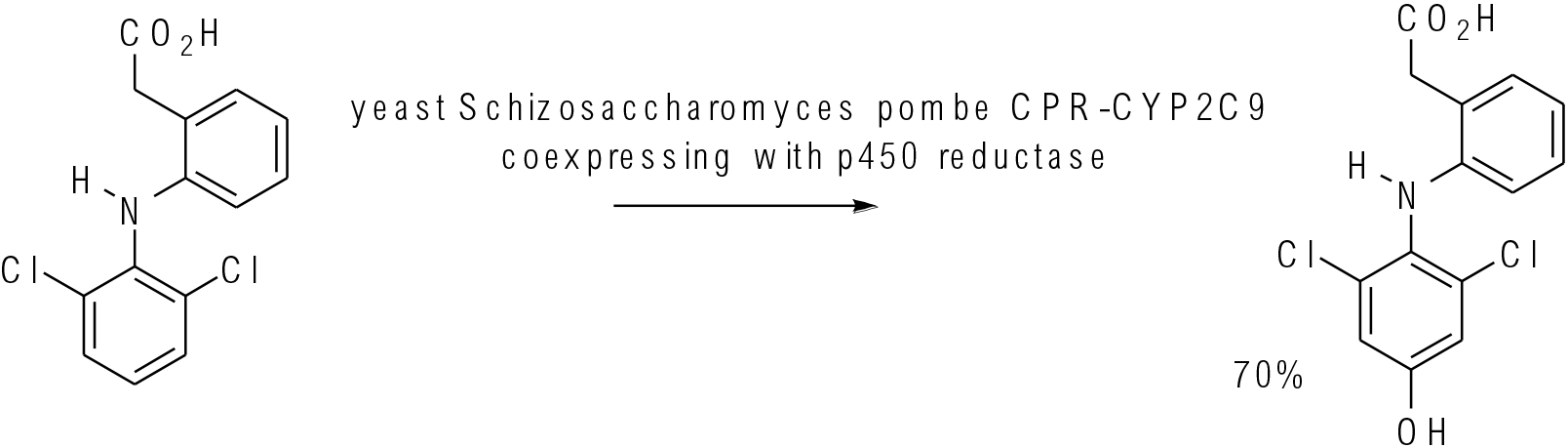
Appl. Biochem. Biotechnol. 2011, 163, 965–980
10 g scale
Green Review
-
Atom efficiency (by-products, molecular weight)
Catalytic technology—but enzyme loading should be optimized and steps using stoichiometric reagents like cofactor recycle should be optimized to avoid unnecessary excess of reagents. - Safety Concerns
Generally considered a safe technology to scale-up. Issues—enzymes/proteins can be sensitizers by inhalation, and some by mode of action can be skin irritants (proteases). If viable GMO cells are used, local regulations relating to use of GMOs need to be followed. - Toxicity and environmental/aquatic impact
No real concerns—enzymes are non-toxic and readily biodegradable. If viable GMO cells are used, these need to be passivated before discharge into the environment. - Cost, availability & sustainable feedstocks
Enzymes are produced from natural sustainable feedstocks. - Sustainable implications
Enzymes are made via fermentation; cloned and recombinant enzymes would be at no risk from depletion. Enzymes break down in the environment and the constituent amino acids are recycled in nature. The use of over expressed recombinant enzymes is generally much better than natural enzymes on a life cycle impact basis.