Nitrilase/nitrile Hydrases
Mechanism + Description
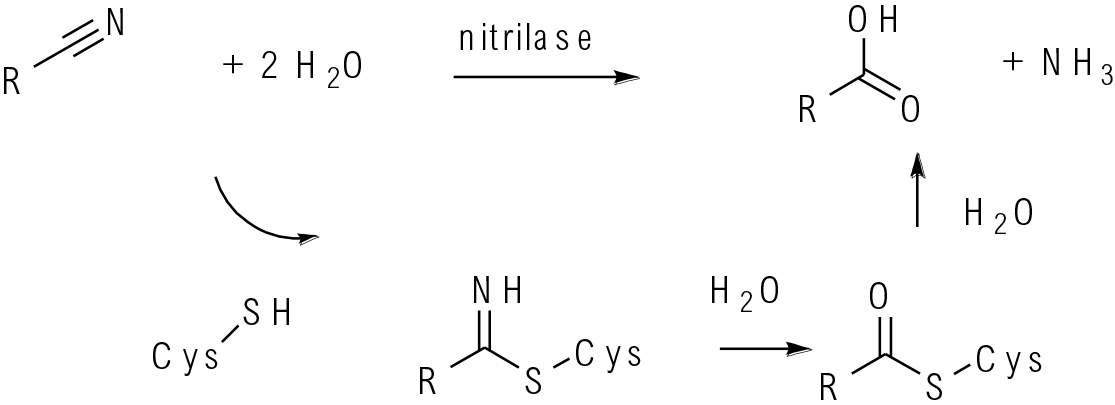
Nitrilase enzymes use the lys-cys-glu catalytic triad. The nitrile is hydrolysed to imidoyl thioester by cysteine in the active site. Reaction with water gives a thioester which is further hydrolysed to the product. Nitrile hydratases have a coordinated metal in the active centre usually Co or Fe bound to cysteine and other amino acids. Binding of the nitrile to the metal centre activates the nitrile to the addition of one mole of water.
General comments
Nitrilase and nitrile hydratase enzymes are used for the selective manipulation of nitriles. Both enzymes will selectively manipulate nitriles in the presence of other hydrolysable functionality such as esters, amides, etc. Nitrile hydratases add only one molecule of water generating the carboxamide exclusively. Nitrilases add two moles of water giving the carboxylate and ammonia. In nature, hydratases are often co-expressed with amidases which hydrolyse the subsequent carboxamide to the carboxylic acid. Both classes of enzyme can be used for chiral chemistry—kinetic resolutions, DKRs and for the selective hydrolysis of nitriles avoiding strong acids and bases that could generate large amounts of waste or destroy sensitive molecules.
Key references
Relevant scale up examples
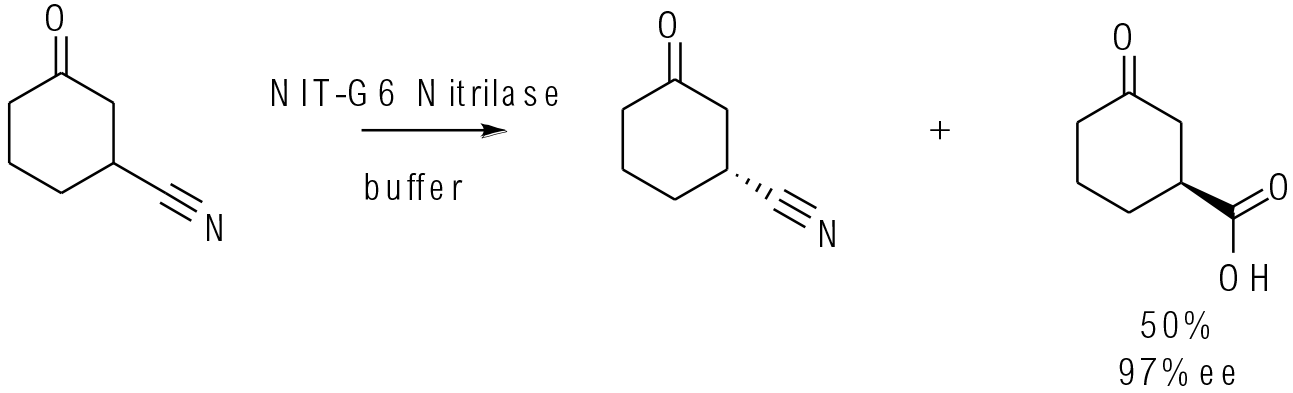
Org. Process Res. Dev. 2018, 22, 871−879
20 g scale
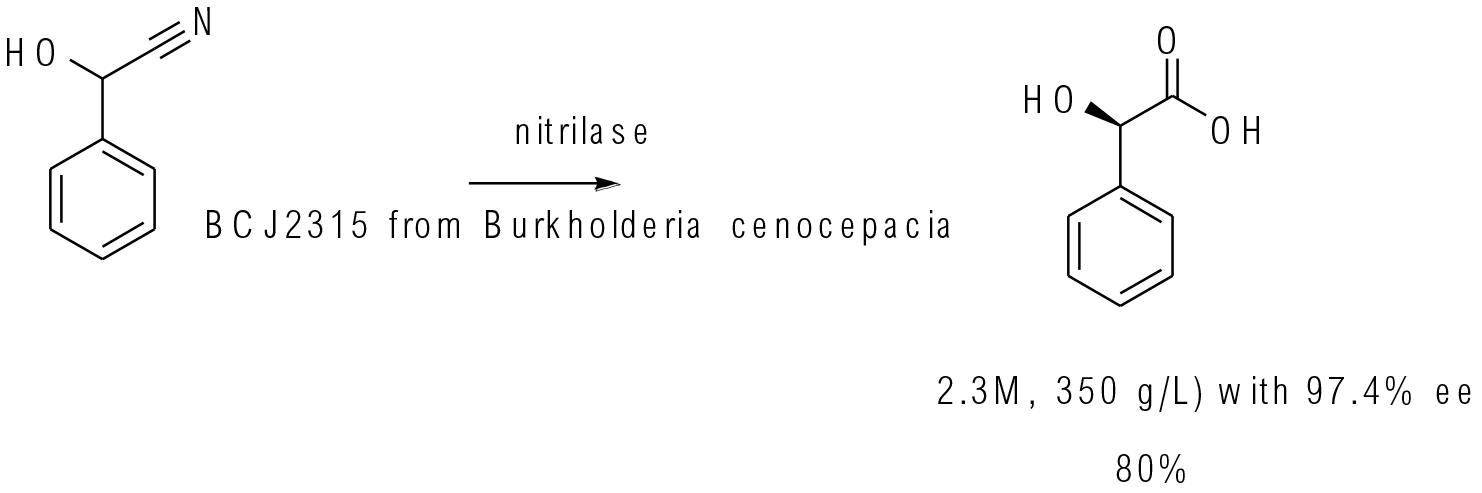
Org. Process Res. Dev. 2015, 19, 2012−2016
500 g scale
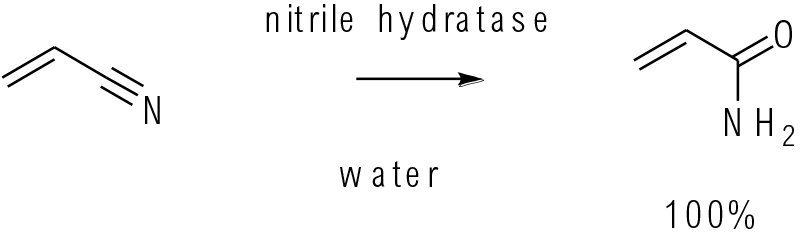
Pure & App. Chem. 1990, 62 (7), 1441−1444
100s kg scale
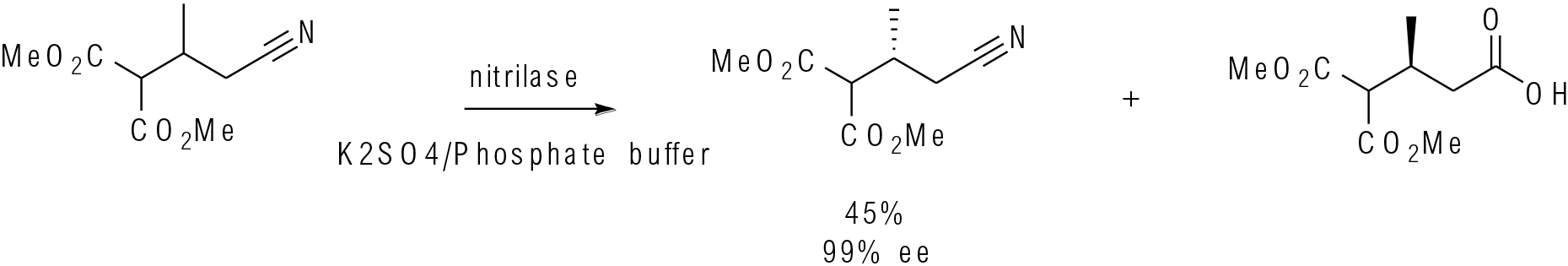
Org. Lett. 2017, 19, 4806−4809
200 g scale
Green Review
-
Atom efficiency (by-products, molecular weight)
Catalytic technology—but enzyme loading should be optimized and steps using stoichiometric reagents like cofactor recycle should be optimized to avoid unnecessary excess of reagents. - Safety Concerns
Generally considered a safe technology to scale-up. Issues—enzymes/proteins can be sensitizers by inhalation, and some by mode of action can be skin irritants (proteases). If viable GMO cells are used, local regulations relating to use of GMOs need to be followed. - Toxicity and environmental/aquatic impact
No real concerns—enzymes are non-toxic and readily biodegradable. If viable GMO cells are used, these need to be passivated before discharge into the environment. - Cost, availability & sustainable feedstocks
Enzymes are produced from natural sustainable feedstocks. - Sustainable implications
Enzymes are made via fermentation; cloned and recombinant enzymes would be at no risk from depletion. Enzymes break down in the environment and the constituent amino acids are recycled in nature. The use of over expressed recombinant enzymes is generally much better than natural enzymes on a life cycle impact basis.