Ene Reductases
Mechanism + Description
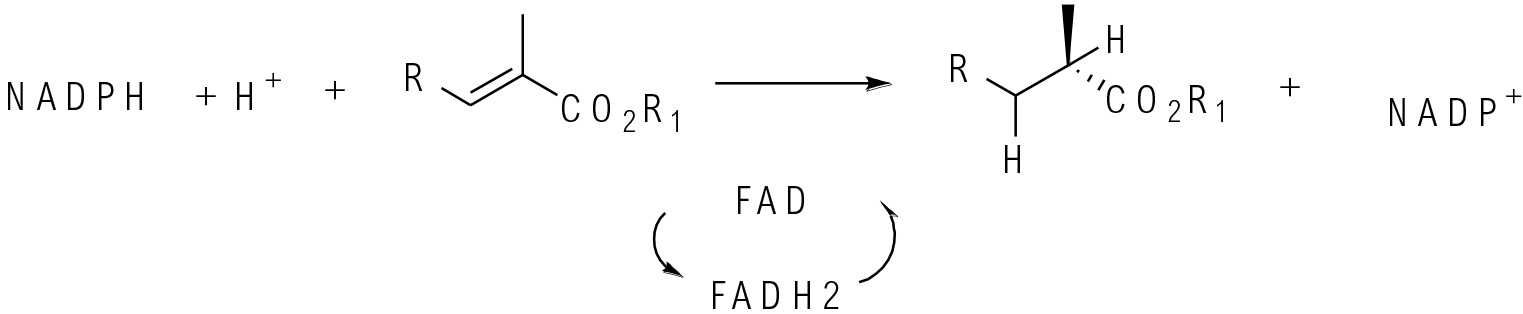
Alkene reductases (ene reductase) simultaneously deliver a hydride and proton in a trans fashion to an alkene double bond. The hydride comes from a reduced flavin cofactor. The oxidised cofactor is reduced back by NAD(P)H.
General comments
Ene reductases are used to reduce activated alkene bonds in a chiral fashion. Generally, the alkene needs to be activated by an electron-withdrawing group like COR2, CN, PO(OR)2, NO2, COR, CHO, etc. In the absence of an activating group, little or no reduction is seen. Traditionally ene reductase-type reductions were performed by enzymes related to old yellow enzyme and related enzymes in yeasts, but many mutants now exist, and most reductions are run with isolated enzymes rather than whole cells. Ene reductases tend to be selective for akene reduction and carbonyl groups in the molecule are not reduced. Since the oxidised flavin cofactor needs to be reduced back to continue the catalytic cycle, a robust NAD(P)H recycle system needs to be in place.
Key references
Relevant scale up examples

Org. Process Res. Dev. 2018, 22, 871−879
40 g scale

J. Chem. Tech. Biotechnol. 1997, 68, 324−330
20 g scale

Org. Process Res. Dev. 2012, 16, 269-276
1 g scale
Green Review
-
Atom efficiency (by-products, molecular weight)
Catalytic technology—but enzyme loading should be optimized and steps using stoichiometric reagents like cofactor recycle should be optimized to avoid unnecessary excess of reagents. - Safety Concerns
Generally considered a safe technology to scale-up. Issues—enzymes/proteins can be sensitizers by inhalation, and some by mode of action can be skin irritants (proteases). If viable GMO cells are used, local regulations relating to use of GMOs need to be followed. - Toxicity and environmental/aquatic impact
No real concerns—enzymes are non-toxic and readily biodegradable. If viable GMO cells are used, these need to be passivated before discharge into the environment. - Cost, availability & sustainable feedstocks
Enzymes are produced from natural sustainable feedstocks. - Sustainable implications
Enzymes are made via fermentation; cloned and recombinant enzymes would be at no risk from depletion. Enzymes break down in the environment and the constituent amino acids are recycled in nature. The use of over expressed recombinant enzymes is generally much better than natural enzymes on a life cycle impact basis.