HCN Lyase
Mechanism + Description
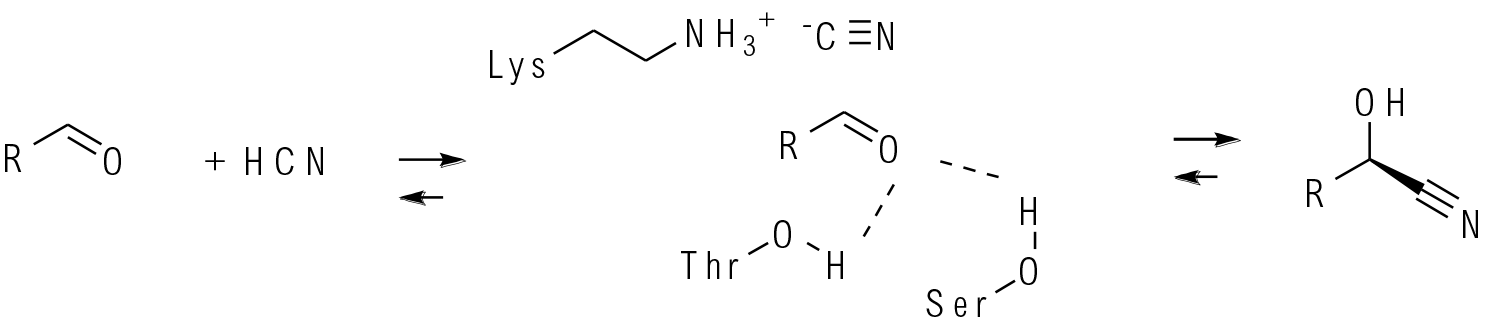
HCN lyases are members of the α/β-hydrolase superfamily and will catalyse the formation of C-C bonds from carbonyl compounds and cyanide. The carbonyl is activated by hydrogen bonding and the nitrile is held in close proximity by a protonated lysine. Chirality is generated by addition of the nitrile to one prochiral face of the carbonyl.
General comments
Chiral hydroxy nitriles are very versatile intermediates for conversion to other synthons such as α-hydroxyl acids. HCN lyases catalyse the chiral addition of cyanide to aldehydes and ketones. The reaction is an equilibrium and tends to lie on the side of products when aldehydes are substrates, and on the side of starting materials when ketones are used. Both R and S selective enzymes are known, and many have been improved by evolution. A variety of cyanide sources can be used, but simple HCN usually produces the best results, although needs appropriate care when used on any scale. Key to the success of a chiral synthesis is to supress any non-catalysed background addition which would degrade the product ee. HCN lyases are often used in combination with other enzymes like nitrilases or nitrile hydratases to convert the product hydroxynitrile into acids or amides thus making the reaction irreversible.
Key references
Relevant scale up examples
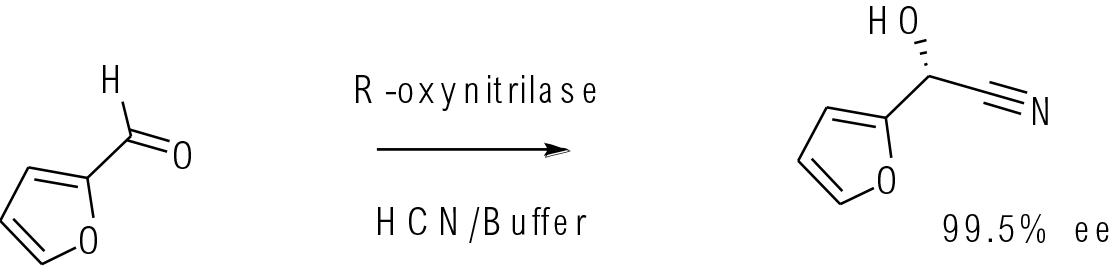
Org. Process Res. Dev. 2006, 10, 618–621
30 g scale
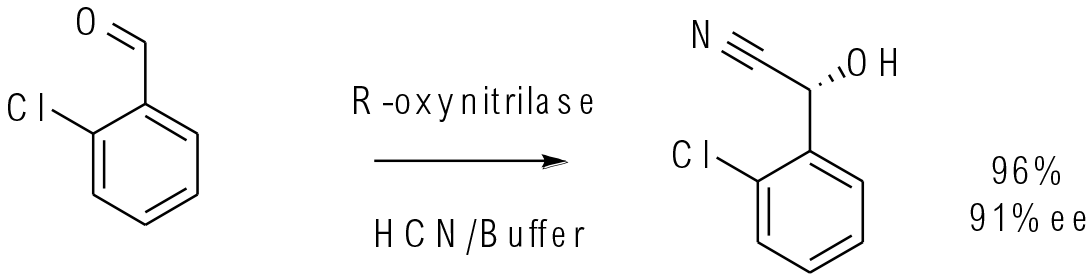
Org. Process Res. Dev. 2003, 7, 828–831
25 g scale
Green Review
-
Atom efficiency (by-products, molecular weight)
Catalytic technology—but enzyme loading should be optimized and steps using stoichiometric reagents like cofactor recycle should be optimized to avoid unnecessary excess of reagents. - Safety Concerns
Generally considered a safe technology to scale-up. Issues—enzymes/proteins can be sensitizers by inhalation, and some by mode of action can be skin irritants (proteases). If viable GMO cells are used, local regulations relating to use of GMOs need to be followed. - Toxicity and environmental/aquatic impact
No real concerns—enzymes are non-toxic and readily biodegradable. If viable GMO cells are used, these need to be passivated before discharge into the environment. - Cost, availability & sustainable feedstocks
Enzymes are produced from natural sustainable feedstocks. - Sustainable implications
Enzymes are made via fermentation; cloned and recombinant enzymes would be at no risk from depletion. Enzymes break down in the environment and the constituent amino acids are recycled in nature. The use of over expressed recombinant enzymes is generally much better than natural enzymes on a life cycle impact basis.
Green Review
-
Atom efficiency (by-products, molecular weight)
Catalytic technology—but enzyme loading should be optimized and steps using stoichiometric reagents like cofactor recycle should be optimized to avoid unnecessary excess of reagents. - Safety Concerns
Generally considered a safe technology to scale-up. Issues—enzymes/proteins can be sensitizers by inhalation, and some by mode of action can be skin irritants (proteases). If viable GMO cells are used, local regulations relating to use of GMOs need to be followed. - Toxicity and environmental/aquatic impact
No real concerns—enzymes are non-toxic and readily biodegradable. If viable GMO cells are used, these need to be passivated before discharge into the environment. - Cost, availability & sustainable feedstocks
Enzymes are produced from natural sustainable feedstocks. - Sustainable implications
Enzymes are made via fermentation; cloned and recombinant enzymes would be at no risk from depletion. Enzymes break down in the environment and the constituent amino acids are recycled in nature. The use of over expressed recombinant enzymes is generally much better than natural enzymes on a life cycle impact basis.