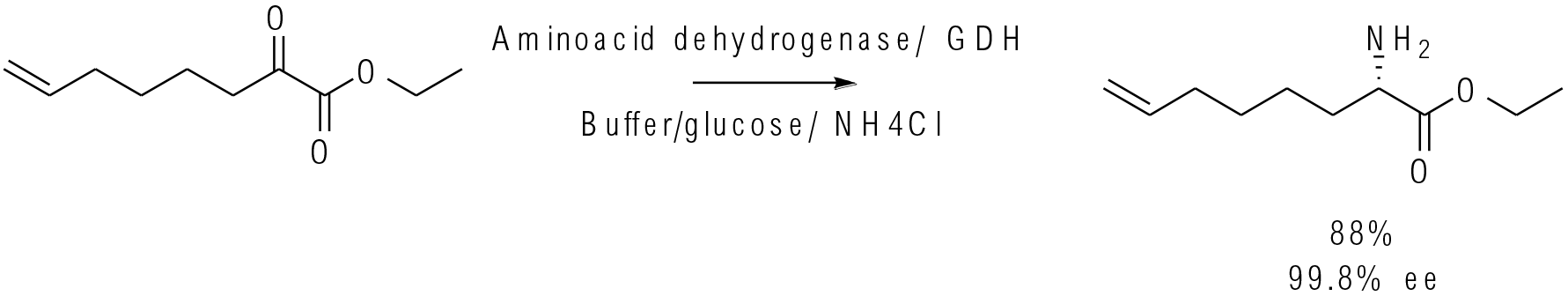Mechanism + Description
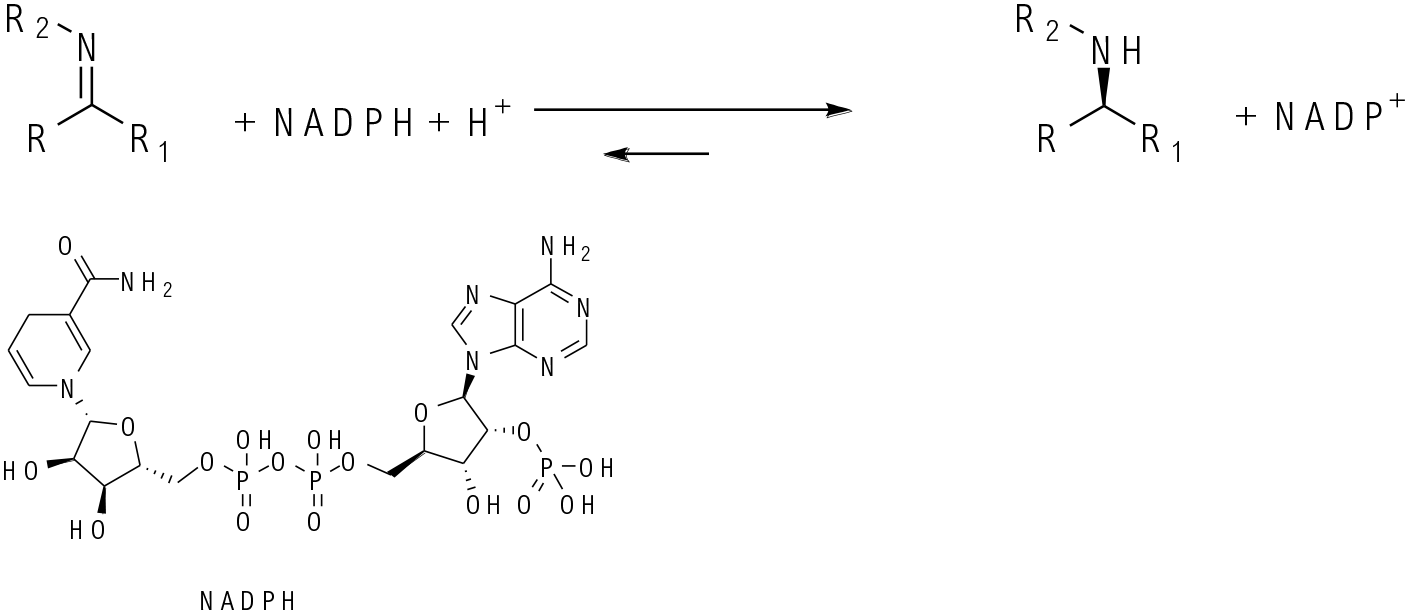
General comments
Related enzymes both producing chiral amines and requiring NAD(P)+ – NAD(P)H cofactor recycling systems to ensure an efficient catalytic cycle. IREDs reduce pre-formed imines – usually cyclic since the cyclic imine is preferred in the mainly aqueous systems. Amine dehydrogenases will reduce imines formed in situ and will work with non-cyclic systems. Typically, an NH3 source is needed like NHM4Cl. In general, ADH/KRED enzymes will not reduce imines, and most ketones are not reduced by IRED/amine dehydrogenase enzymes, but recently some exceptions have been reported.
Key references
Mangas-Sanchez, J.; France, S. P.; Montgomery, S. L.; Aleku, G. A.; Man, H.; Sharma, M.; Ramsden, J. I.; Grogan, G.; Turner, N. J. Imine reductases (IREDs). Curr. Opin. Chem. Biol. 2017, 37, 19–25.
Hyslop, J. F.; Lovelock, S. L.; Watson, A. J. B.; Sutton, P. W.; Roiban, G.-D. N-Alkyl-α-amino acids in Nature and their biocatalytic preparation. J. Biotechnol. 2019, 293, 56–65.
Hyslop, J. F.; Lovelock, S. L.; Sutton, P. W.; Brown, K. K.; Watson, A. J. B. Roiban, G.-D. Biocatalytic Synthesis of Chiral N-Functionalized Amino Acids. Angew. Chem. Int. Ed. 2018, 57, 13821–13824.
Maugeri, Z.; Rother, D. Reductive amination of ketones catalyzed by whole cell biocatalysts containing imine reductases (IREDs). J. Biotechnol. 2017, 258, 167–170.
Grogan, G. Synthesis of chiral amines using redox biocatalysis. Curr. Opin. Chem. Biol. 2018, 43, 15–22.
Knaus, T.; Böhmer, W.; Mutti, F. G. Amine dehydrogenases: efficient biocatalysts for the reductive amination of carbonyl compounds. Green Chem. 2017, 19, 453–463.
Hussain, S.; Leipold, F.; Man, H.; Wells, E.; France, S. P.; Mulholland, K. R.; Grogan, G.; Turner, N. J. An (R)-Imine Reductase Biocatalyst for the Asymmetric Reduction of Cyclic Imines. ChemCatChem 2015, 7, 57–5839.
Leipold, F.; Hussain, S.; Ghislieri, D.; Turner, N. J. Asymmetric Reduction of Cyclic Imines Catalyzed by a Whole‐Cell Biocatalyst Containing an (S)‐Imine Reductase ChemCatChem 2013, 5, 3505–3508.
Rodríguez-Mata, M.; Frank, A.; Wells, E.; Leipold, F.; Turner, N. J.; Hart, S.; Turkenburg, J. P.; Grogan, G. Structure and Activity of NADPH‐Dependent Reductase Q1EQE0 from Streptomyces kanamyceticus, which Catalyses the R‐Selective Reduction of an Imine Substrate. ChemBioChem 2013, 14, 1372–1379.
Aleku, G. A.; Man, H.; France, S. P.; Leipold, F.; Hussain, S.; Toca-Gonzalez, L.; Marchington, R.; Hart, S.; Turkenburg, J. P.; Grogan, G.; Turner, N. J. Stereoselectivity and Structural Characterization of an Imine Reductase (IRED) from Amycolatopsis orientalis. ACS Catal. 2016, 6, 3880–3889.
Scheller, P. N.; Lenz, M.; Hammer, S. C.; Hauer, B.; Nestl, B. M. Imine Reductase‐Catalyzed Intermolecular Reductive Amination of Aldehydes and Ketones. ChemCatChem 2015, 7, 3239–3242.
Yin, X.; Liu, Y.; Meng, L.; Zhou, H.; Wu, J.; Yang, L. Rational Molecular Engineering of Glutamate Dehydrogenases for Enhancing Asymmetric Reductive Amination of Bulky Alpha -Keto Acids. Adv. Synth. Catal. 2019, 361, 803–812.
Tseliou, V.; Masman, M. F.; Böhmer, W.; Knaus, T.; Mutti, F. G. Mechanistic Insight into the Catalytic Promiscuity of Amine Dehydrogenases: Asymmetric Synthesis of Secondary and Primary Amines. ChemBioChem 2019, 20, 800–812.
Abrahamson, M. J.; Wong, J. W.; Bommarius, A. S. The Evolution of an Amine Dehydrogenase Biocatalyst for the Asymmetric Production of Chiral Amines. Adv. Synth. Catal. 2013, 355, 1780–1786.
Relevant scale up examples

Org. Process Res. Dev. 2019, 23, 1262–1268
25 kg scale
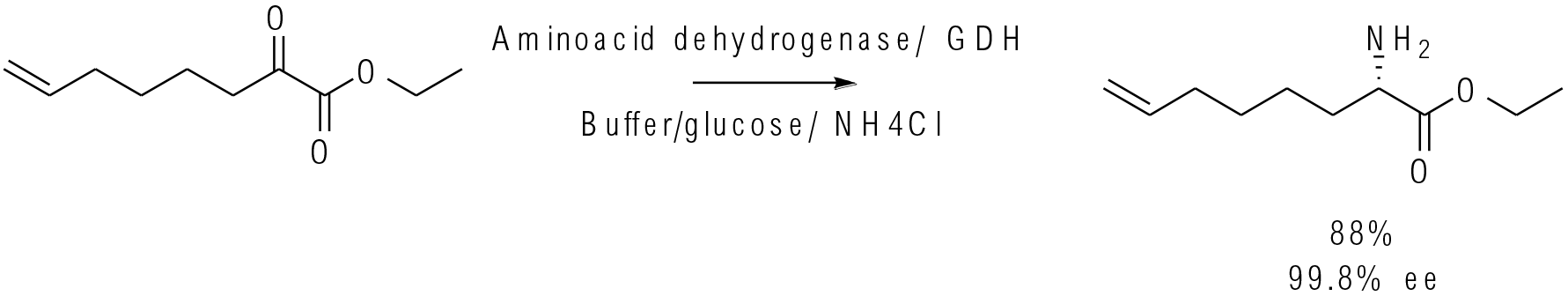
Org. Process Res. Dev. 2016, 20, 76−80
20 g scale
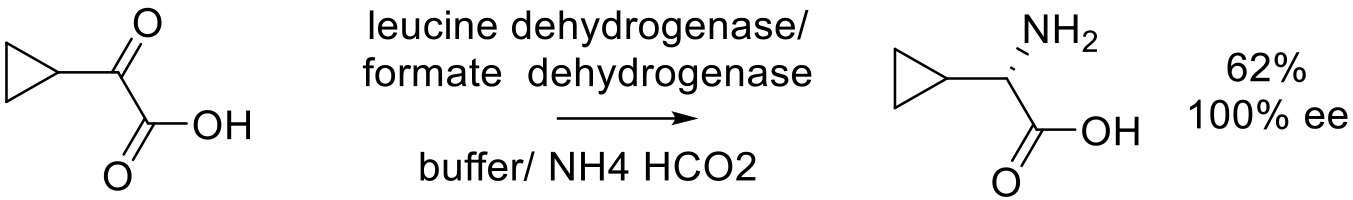
Org. Process Res. Dev. 2012, 16, 464−469
50 g scale
Green Review
-
Atom efficiency (by-products, molecular weight)
Catalytic technology—but enzyme loading should be optimized and steps using stoichiometric reagents like cofactor recycle should be optimized to avoid unnecessary excess of reagents.
- Safety Concerns
Generally considered a safe technology to scale-up. Issues—enzymes/proteins can be sensitizers by inhalation, and some by mode of action can be skin irritants (proteases). If viable GMO cells are used, local regulations relating to use of GMOs need to be followed.
- Toxicity and environmental/aquatic impact
No real concerns—enzymes are non-toxic and readily biodegradable. If viable GMO cells are used, these need to be passivated before discharge into the environment.
- Cost, availability & sustainable feedstocks
Enzymes are produced from natural sustainable feedstocks.
- Sustainable implications
Enzymes are made via fermentation; cloned and recombinant enzymes would be at no risk from depletion. Enzymes break down in the environment and the constituent amino acids are recycled in nature. The use of over expressed recombinant enzymes is generally much better than natural enzymes on a life cycle impact basis.