Bleach (NaOCl) Oxidation
Mechanism + Description
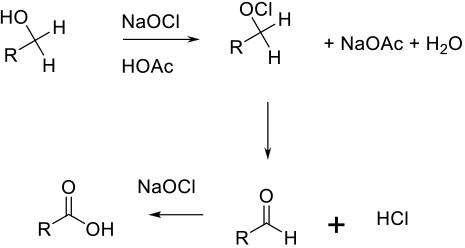 In acidic media, the loss of water with the addition of the OCl- anion, followed by the loss of HCl to generate
the aldehyde that is oxidized to the acid by a similar mechanism.
In acidic media, the loss of water with the addition of the OCl- anion, followed by the loss of HCl to generate
the aldehyde that is oxidized to the acid by a similar mechanism.
General comments
NaOCl is usually used as a terminal/stoichiometric co-oxidant with catalysts that provide a stronger oxidant than HOCl alone. In some cases, NaOCl alone can give the acids of esters from primary alcohols.
Key references
Relevant Scale-Up Examples with Scheme
No scale-up examples identified.