RuCl3/Tetrapropylammonium perruthenate (TPAP) Plus Co-oxidants
Mechanism + Description
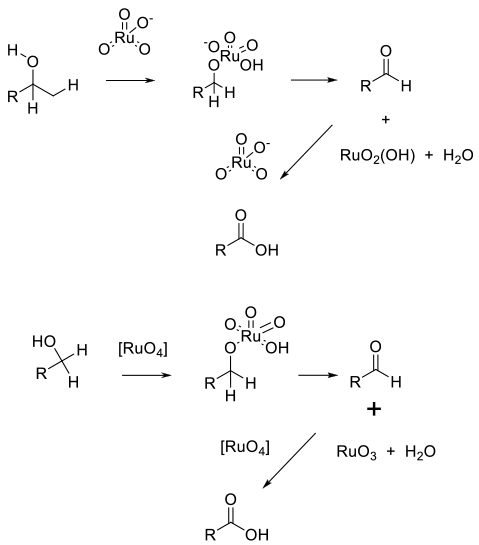 TPAP ( nPr4NRuO4) provides a source of Ru (VII) soluble in a range of organic solvents. Alcohol oxidation takes place via an Ru complex that generates Ru (V) and the product. The catalyst is regenerated using an added terminal oxidant.
TPAP ( nPr4NRuO4) provides a source of Ru (VII) soluble in a range of organic solvents. Alcohol oxidation takes place via an Ru complex that generates Ru (V) and the product. The catalyst is regenerated using an added terminal oxidant.
Ru (III) salts are oxidized in situ by a terminal oxidant, which is the active species. Oxidation takes place via the Ru ester, and the reduced Ru (VI) species is recycled back to RuO4 by the oxidant. In both cases, the aldehyde is oxidized by an identical mechanism via the hydrate.
General comments
Catalytic RuCl3 and TPAP prove milder, more easily controlled, and greener versions of stoichiometric RuO4 oxidation. TPAP gives solubility in a range of organic solvents. TPAP is used catalytically and driven by the addition of a terminal oxidant. The original oxidant, morpholine N-oxide (NMO), gives rise to poor atom economy. Later variants have attempted to address this by using air instead as the co-oxidant. RuCl3 typically uses terminal oxidants like NaIO4, NaOCl, H2O2 to regenerate RuO4. Generally, Ruthenium salts are low impact with low catalytic loadings. As with all heavy metals, the contamination of the environment needs to be avoided and levels in the final API need to be controlled. Other risks depend on the co-oxidant used.
Key references
Relevant Scale-Up Examples with Scheme
None found.
Green Review
-
Atom efficiency (by-products Mwt)
While it has a high Mol. Wt., TPAP is catalytic and can usually be optimized to have minimal effects on mass balance. The major atom efficiency hit is from the reoxidant. N-methyl morpholine N oxide generates a stoichiometric amount of N Methyl morpholine as a by-product. - Safety Concerns
The reactions can be quite exothermic and hard to control in some instances. Small-scale observation and assessment is strongly recommended before scale-up. - Toxicity and environmental/aquatic impact
Generally low impact with low catalytic loadings. As with all heavy metals, contamination of the environment needs to be avoided and levels in the final API need to be controlled. Other risks depend on the co-oxidant used. - Cost, availability & sustainable feedstocks
While TPAP is catalytic, it is also expensive with some limitations on scale. - Sustainable implications
Like most precious metals, Ru has a high LCI and is rated at high risk of depletion.