Nitric Acid
Mechanism + Description
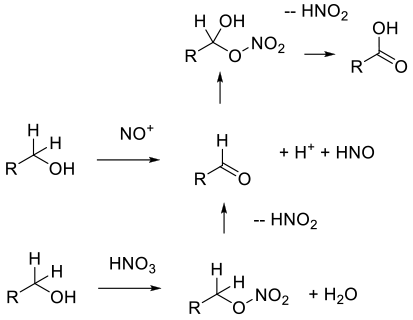 Probably via hydride abstraction to aldehyde,
or formation of a nitrate ester, then elimination
to the aldehyde. The aldehyde is then oxidized to
the carboxylic acid by a number of mechanisms
depending on the structure of the aldehyde, either via
a nitrate ester or a radical mechanism.
Probably via hydride abstraction to aldehyde,
or formation of a nitrate ester, then elimination
to the aldehyde. The aldehyde is then oxidized to
the carboxylic acid by a number of mechanisms
depending on the structure of the aldehyde, either via
a nitrate ester or a radical mechanism.
General comments
Nitric acid is a very cheap and powerful oxidant but is rarely used due to the potential for over-oxidation and nitration side reactions. Other issues are very exothermic reactions and the need to deal with NO(X) off-gasses. However, a number of primary alcohols can be oxidised to carboxylic acids with HNO3. These oxidations need to be scaled with great care due to the exothermic nature and gas output of HNO3 reactions. Continuous processing/flow chemistry may provide a safer alternative to batch or semi-batch reactions with HNO3.
Key references
Alcohol to carboxylic acid with HNO3: Mondanaro, K. R.; Dailey, W. P. 3-Chloro-2-(Chloromethyl)-1-Propene. Org. Synth. 1998,75, 89.
Oxidation Br(CH2)4OH to Br(CH2)3CO2H with HNO3: Degering, E. F.; Boatright, L. G. Studies on the Synthesis of Lysine. J. Am. Chem. Soc. 1950, 72 (11), 5137–5139.
Relevant Scale-Up Examples with Scheme
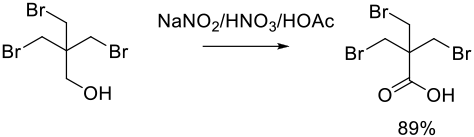
Org. Process Res. Dev. 2011, 15 (3), 581–584.
Experimental
425 kg scale