Potassium/Sodium Permanagnate K(Na)MnO4
Mechanism + Description
The mechanism proceeds via hydride elimination from the alkoxide to give the aldehyde, which is then oxidized to the acid. The second oxidation may be a hydride loss from the aldehyde hydrate or a more complex hydrogen atom extraction process, or via a manganate ester of the hydrate.
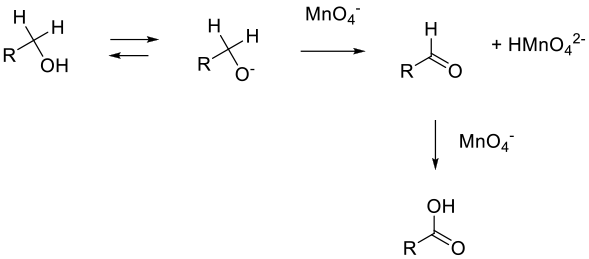
General comments
KMnO4 or NaMnO4 are classical oxidants for the conversion CH2OH-CO2H. MnO2 is selective for oxidation to CHO only. The reaction is often heterogeneous with excess oxidant being used and large quantities of lower valent Mn oxides generated as by-products. KMnO4 is a very reactive and unselective oxidant and may react with other oxidizable groups present in the substrate. Reactions are often performed in water based with added PTC to increase solubility. Often run under basic conditions, this can cause issues with base-sensitive substrates. A background decomposition of permanganate to MnO2 and O2 often means a greater than stoichiometric quantity has to be employed.
Key references
Example of KnMOS4 oxidation: Ciufolini, M. A.; Swaminathan, S. Synthesis of a Model DepsipeptideSegment of Luzopeptins (BBM 928), Potent Antitumor and Antiretroviral Antibiotics. Tetrahedron Lett. 1989, 30 (23), 3027–3028.
Relevant Scale-Up Examples with Scheme
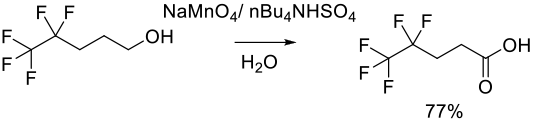
Org. ProcessRes. Dev. 1999, 3 (5), 363−364.
Experimental
2 kg scale
Green Review
- Atom efficiency (by-products Mwt)
Stoichiometric atom efficiency gives Mn oxides and H2O as by-products, but often, a large excess of KMnO4 is needed, so the mass economy is quite poor overall. - Safety Concerns
KMnO4 is not compatible with some solvents, and reactions can be very exothermic. Issues with runaway reactions can occur, especially if run under heterogeneous conditions. - Toxicity and environmental/aquatic impact
Mn is an essential element, but is toxic in higher doses. High concentrations of Mn in aqueous effluent should not be discharged into the environment. KMnO4 is a gross cellular poison. Metal contamination of the final product needs to be considered.
http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q3D/Q3D_Step2b.pdf - Cost, availability & sustainable feedstocks
KMnO4 is readily available and cheap. The generation of Mn oxide residues may incur high solvent use during product isolation and plant cleaning. Recyclable. - Sustainable implications
Mn oxide residues can be recycled; otherwise, disposal is via landfill. All metals have a high LCA impact from mining and refining operations, so use should be catalytic with recovery and recycle. Mn is listed as at moderate risk of depletion from natural resources.