Nickel Peroxide, NiO(OH)2
(includes in-situ preparation from other Nickel salts)
Mechanism + Description
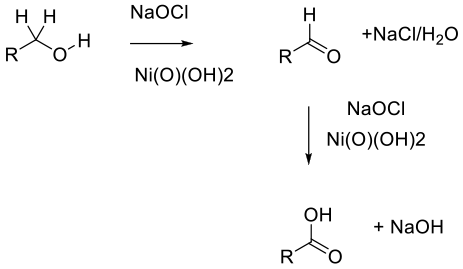 Nickel oxide hydroxide – NiO(OH)2 – appears to act as a heterogeneous reagent commonly generated in-situ from nickel salts such as NiCl2 or Ni(OAc)2, which then results in high surface area nanoparticles (ca. 4 nm). The mechanism is unclear, but evidence suggests radical rather than ionic.
Nickel oxide hydroxide – NiO(OH)2 – appears to act as a heterogeneous reagent commonly generated in-situ from nickel salts such as NiCl2 or Ni(OAc)2, which then results in high surface area nanoparticles (ca. 4 nm). The mechanism is unclear, but evidence suggests radical rather than ionic.
General comments
The actual active nickel substrate (nickel peroxide is commonly termed, though it is probably NiO(OH)2) was originally used in molar excess. Recently, however, catalytic variants are more prominent and add utility on scale. The oxidation of primary alcohols to carboxylic acids, secondary alcohols to ketones, aldehydes to carboxylic acids, and α,β-unsaturated carboxylic acids to epoxy acids is demonstrated by using 2.5 mol percent of the nickel catalyst and common bleach as the terminal oxidant. Aldehydes are intermediates, and isolated aldehydes can also be used as substrates. Reactions using/giving liquid reagents/products can be run solvent-free.
Key references
Relevant Scale-Up Examples with Scheme
No scale-up examples identified.